Hello Community,
I would like to share some key insights from recent developments at Dr. Reddy’s Laboratories Limited, as it’s been a while since we had a detailed update. Below are highlights from the AR 2024, AGM, Q1 FY25 Results, and the latest credit rating report.
Business Divisions and Products:
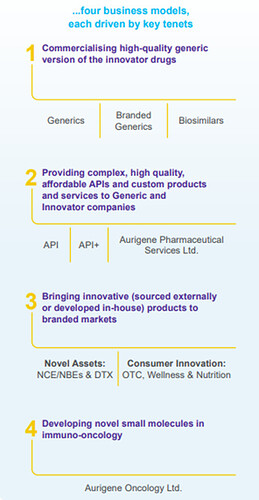
DRL provides a wide range of pharmaceutical products and services, including:
- Generics
- Active Pharmaceutical Ingredients (APIs)
- Custom Pharmaceutical Services
- Biosimilars
- Differentiated Formulations
The company is organized into three divisions:
- Global Generics– Contributed to 88% of revenues in FY2024.
- Pharmaceutical Services and Active Ingredients (PSAI)– Contributed 11%.
- Others – Made up 1% of revenues.
Key therapeutic areas include treatments for the central nervous system, gastrointestinal issues, oncology, cardiovascular diseases, and pain management. DRL’s primary markets are the U.S., India, Western Europe, Russia, and CIS5 nations.
Manufacturing and R&D:
API Manufacturing Facilities: DRL operates nine facilities, including six in India, one in Mexico, one in the U.S., and one in the U.K.
Formulation Manufacturing Facilities: The company has 13 plants in India, and one each in the U.S. and China.
Biologics Facility: DRL operates with one biological facility in India.
R&D Centers: It has eight technology development and research centers worldwide, supported by a team of 3,335 R&D scientists.
Product and Regulatory Achievements:
New Product Launches: DRL has launched 181 new products globally, with 20 in North America, 42 in Europe, 106 in emerging markets, and 13 in India.
Drug Master Files (DMFs): It has filed 133 new DMFs globally, including 11 in the U.S. The cumulative global DMFs stand at 1,537, with 251 in the U.S.
Regulatory Filings: DRL has filed 241 dossiers, including Abbreviated New Drug Applications (ANDAs) and New Drug Applications (NDAs). Out of these, 86 filings are pending approval, comprising 81 ANDAs and 5 NDAs. Of these, 50 are Para IV filings, and the company believes 24 hold “First-to-File” status.
Company Value creations and focus
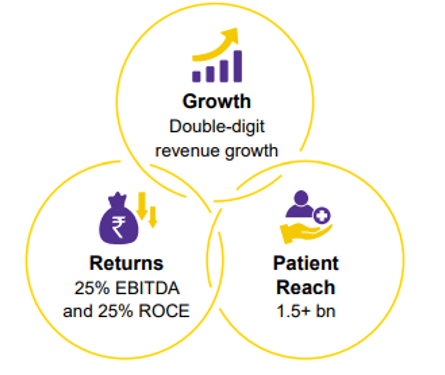
DRL aspires to generate double-digit revenue growth, 25% of EBITDA & ROCE, and to serve 1.5Bn patients in the next 5-6 years.
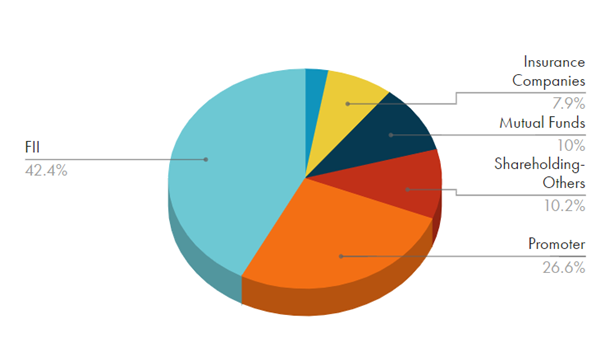
Share Holding Pattern: Public Holding is 10%.
In FY2024, DRL was ranked as the 8th largest generic pharmaceutical company in the U.S. by sales. Globally, it was ranked 11th in India and 15th in Russia as per IQVIA data. The company has grown its market share in several products and improved its ranking in key therapeutic areas across markets.
Aurigene Oncology Limited (AOL)
DRL’s wholly-owned subsidiary, Aurigene Oncology Limited (AOL), is focused on discovering and developing novel, best-in-class therapies for cancer and inflammatory diseases, further expanding DRL’s reach in cutting-edge medical research and innovation.
Over the last four decades, DRL has transformed from a company focused on APIs to one that is a leader in formulations, biosimilars, consumer healthcare, and digital therapeutics, positioning itself for continued success in the evolving global pharmaceutical landscape.
I will be sharing additional key highlights in future posts, focusing on the factors driving growth, the identified risks, and the quantitative aspects of the company.