Opportunities
• Botulinum Toxin Indian Market Size is 120 Crore at the moment (Potential is huge in India and international market as well but it is not easy)
• Botulinum Toxin International Market Size 8 Billion and USA 5 Billion
• Critical Care India Market size is 181000 Crores in India
• Infertility is around close to 4000 crores in India and growing 15% yoy
• Dual Chamber Bag initially targeting Anti-infectives. Addressable market size is ~Rs. 3,000 crores in India
• Dydrogesterone product size for this product is ~Rs. 800+ crores growing at 60% YoY in India
• Critical Care around 36000 Crores to 38000 Crores
• D29 Market size 6 million in India
• Sparsh market size is ~Rs. 9,500 crores, growing at a CAGR of 12%
Updates on Capex
- Indore Plant supposed to be Live on September as directed by Management
– Management is walking the talk as the plant is now LIVE and they have started investing in R&D and started to plan on validating Batches
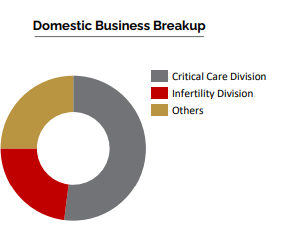
Critical Care Division
- Anti-Bacterial (Penicillin – based Finished Dosage Forms) – Still Maintaining LEADERSHIP POSITION
- Anti-Fungal – Still Maintaining LEADERSHIP POSITION
Approvals
- DCGI Approval received for manufacturing and marketing of Biapenem Dual Chamber Bag
- DCGI approval received to conduct Phase III Clinical trials for Thymosin Alpha injection in
Sepsis – it is a large market
Launched
- Launched Sparsh sub division under Critical Care Division – This will use the most advance technology to smoothen the supply chain process for delivering 100+ high quality injectable products to untapped hospitals and nursing homes including the sub-urban and rural market. Addressable market size is ~Rs. 9,500 crores, growing at a CAGR of 12%
Yet To Launch
- Planning to launch Ceftazidime + Avibactam soon. Gufic will be the only Indian Company to launch this product other than the innovator with inhouse manufactured API (Avibactam)
- Launching the novel ‘Once a week’ anti-infective – Dalbavancin for the 1st time in India in Q4 FY23
In Developement/Clinical Phase :
- Isavuconazole oral option to compliment the injectable by Q3 FY23. The overall market of this molecule is growing at 100%
- A wide range of products being developed in the new drug delivery system of Dual Chamber Syringes which will ensure ease of reconstitution,accurate dosing and maintain
terility from plant to patient - The API Research Development at Navsari has made noteworthy progress in development of molecules in therapeutic categories such as Antifungal, Anticoagulant, Tetracycline Antibiotics, Progestin, Beta 3 adrenegic agonists, Antidiabetic, Cyclopeptides Hormones. These development projects are all progressing in line with the plan
Investment/Partnership
NA
Ferti Care Division
- Maintained 2nd Rank in the high growing Cetrorelix market and increasing its penetration for Enoxaparin in the infertility segment
- Puregraf (HMG) and Puretrig (HCG) continues to register high double-digit growth for female infertility hormones
Approvals
- DCGI approval received to conduct Phase III Clinical trials for Thymosin Alpha injection in Endometriosis
Launched
- Dydrogesterone: This product has been launched. Further, to de-risk the short supply of API, Gufic has vertically integrated to manufacture its own API. Market size for this product is ~Rs. 800+ crores growing at 60% YoY
Yet To Launch
NA
In Developement/Clinical Phase :
NA
Investment/Partnership
- Gufic has invested to develop recombinant alternatives to the urinary source of certain hormones which are critical in the treatment of infertility and thereby ensuring we will be independent of geopolitical as well as currency exchange risks and potential pitfalls in
the next 12-18 months
Healthcare, Stellar & Spark Division
- Sallaki continues to be the market leader in Boswellia Serrata market
Approval
- NA
Launched
- A new multivitamin formulation has been launched which should do well in the coming quarters aiding growth in this segment
- Launched a cannabis extract based topical solution for muscular and arthritic pain relief
Yet to launch
NA
In Developement/Clinical Phase :
- We initiated trial of a new product made from an Indian gum by a standardized extraction process for use in the management of asthma
- Initiated development of a unique liposomal iron formulation
Investment/Partnership
NA
Aestherderm Division
- Stunnox continues to increase penetration in the market.
- Started the training center for new therapies with combination of machines and use of fillers and Botulinum Toxin for face and body contouring
Approval
NA
Launched
NA
Yet to launch
NA
In Developement/Clinical Phase :
- Break-through in the development of novel topical formulation of Botulinum Toxin for the first time in the world
- Developing fillers for Stunnox to complement and complete this basket
Investment/Partnership
• Gufic has partnered with Indian College of Cosmetic Gynaecology (ICCG) in the field of cosmetic vaginal tightening and rejuvenation and organized trainings to promote the use of Botulinum Toxin for these indications
Selvax
Approval
NA
Launched
NA
Yet to launch
NA
In Developement/Clinical Phase :
- The Selvax immunotherapy demonstrated promising results (100% long-term cures alongside induction of protective immunity) in the two pancreatic cancer models tested in the pre-clinical stage. These results align with the other different mouse tumour models tested
- Moreover, it has consistently outperformed FDA approved checkpoint inhibitors which have become first line therapies for some cancers, including melanoma. These results indicate that the Selvax immunotherapeutic approach could offer a viable alternative to existing therapiesfor the treatment of pancreatic cancer
- Current treatment options for pancreatic cancer include surgery, chemotherapy, radiotherapy, and ablation. These options are rarely effective, and in most cases are used to manage symptoms rather than eradicate disease, highlighting a dire need for new treatments that are effective at combating a cancer that is currently incurable
Investment/Partnership
NA
Domestic Business
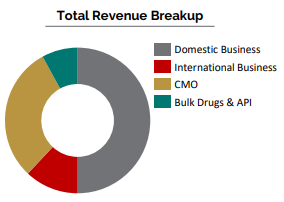
International Business
- 25% Growth in International Business
- 150+ products are in pipeline for registration in over 40 Countries
- Received 2 new product approvals received from each, UK-MHRA and ANVIZA Brazil.
- Received 1 product approval from Health Canada
Awaiting to hear more in CONCALL on Monday 14th November 2022
| Subscribe To Our Free Newsletter |

