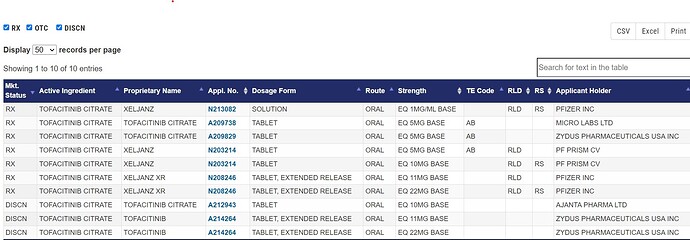
Zydus receives final approval for Tofacitininib 5 mg tablet and tentative approval for 10 mg.
It’s also eligible for 180 days of shared exclusivity for 5 mg strength.
Tofacitininib is a generic version of Pfizer”s XELJANZ which acts by inhibiting Janus kinases enzymes involved in the pathway of inflammatory process.
XELJANZ is indicated in patients with ankylosing spondylitis, moderate to severe rheumatoid arthritis, active psoriatic arthritis and moderate to severe active ulcerative colitis which are autoimmune inflammatory diseases.
Tofacitinib 5mg and 10 mg had annual sales of 900 mn in USA.(worldwide annual sales of 2.3 billion USD).
US FDA orange book shows few more approvals including Ajanta pharma and Micro Labs.
Ajanta pharma seems to have exclusivity for 10 mg tab as per below document of FDA.
Discl: invested
| Subscribe To Our Free Newsletter |