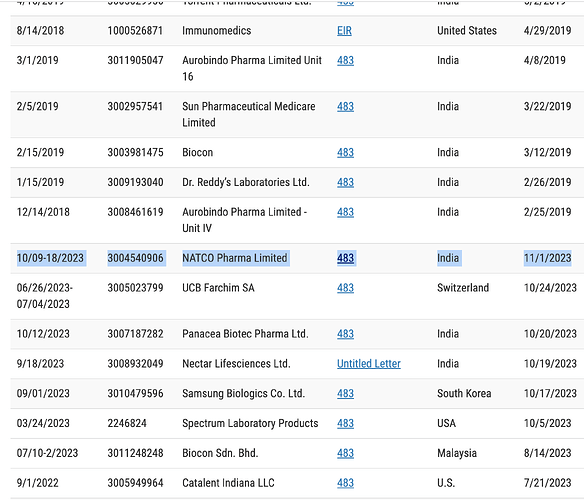
If you are motivated, go to this link
And download the 483 report by searching for Natco
There are few very serious observations made by FDA.
This means that if they have issues in a given batch, then operator can simply delete the records.
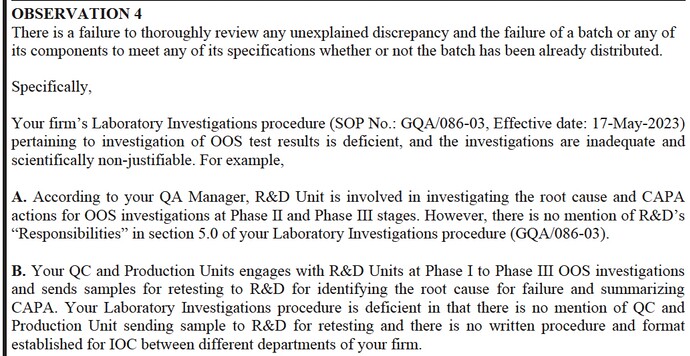
Out of specification issues have been Indian pharma bugbear for 10 years now, its crazy how the industry hasn’t yet resolved these. I understand from a business perspective, why its hard to reject and throw away batches which consume high cost API, but from a safety view point, its a huge issue. And I have seen this observation in atleast 10 other Indian companies.
This is another long term problem, of not maintaining written records.
If you want a summary of these issues, we did a very long form conversation with Amit Rajan who provided amazing insights into how to understand compliance.
Learning pharma compliance with Amit Rajan
Disclosure: Not invested in Natco (no transactions in last-30 days)
| Subscribe To Our Free Newsletter |