Financial Performance (H1 FY24):
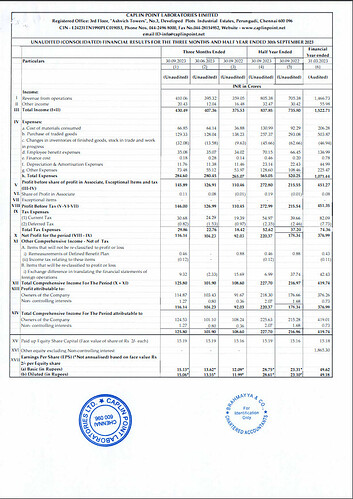
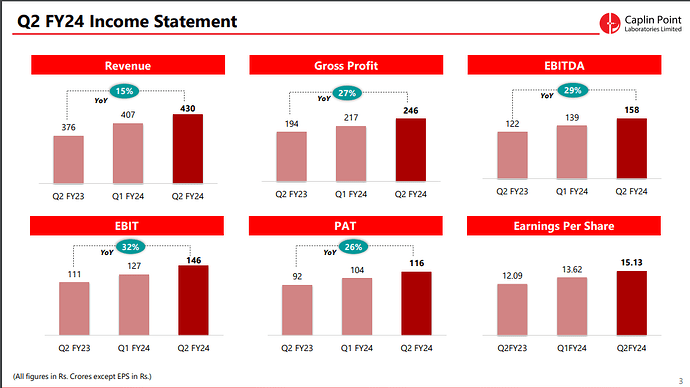
- Operating Revenue: The operating revenue for H1 FY24 reached ₹805.38 Crores, showing a substantial increase of 14.2% compared to the same period last year.
- Profit After Tax (PAT): The PAT for H1 FY24 stood at ₹220.37 Crores, demonstrating a notable year-on-year increase of 23.6%.
- Earnings per Share: The company achieved Earnings per Share (EPS) of ₹28.75.
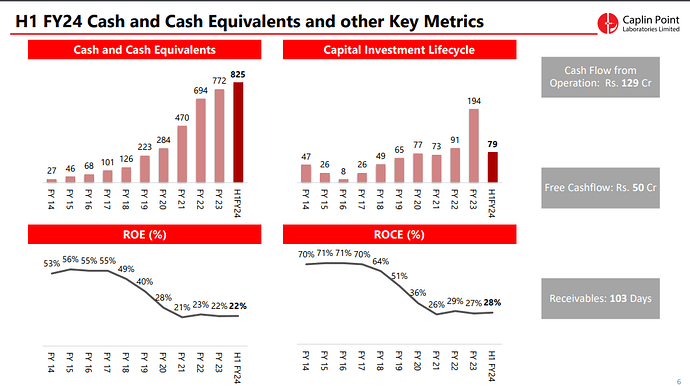
- Cash and Cash Equivalents: Caplin Point maintained a strong financial position with ₹825.4 Crores in cash and cash equivalents.
Financial Highlights (H1 & Q2 FY24):
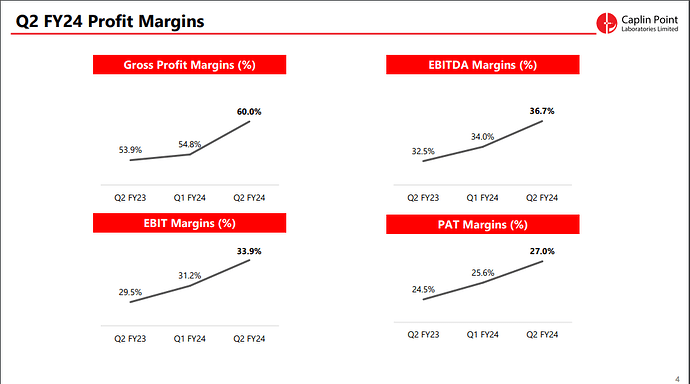
- Gross Margin: The gross margin improved to 60.0% in Q2 FY24 compared to 53.9% in Q2 FY23. For H1 FY24, the gross margin was 57.4%, up from 54.3% in H1 FY23. This improvement was attributed to new product launches across existing and new markets.
- EBITDA Margin: EBITDA margin increased to 36.7% in Q2 FY24 from 32.5% in Q2 FY23. For H1 FY24, it was 35.4%, up from 32.4% in H1 FY23.
- Basic EPS: The Basic EPS increased by 23.3% to ₹28.75 in H1 FY24 compared to ₹23.31 in H1 FY23.
- Cash Flow: The company reported ₹129 Crores in cash flow from operations for H1 FY24.
- Free Cash Flow: After capital expenditure (Capex) investments of ₹79 Crores, the free cash flow was ₹50 Crores.
- Revenue Segments: Latin America and Rest of the World contributed to 85% of the geographical revenues, while the US accounted for 15%.
- Caplin Steriles Ltd (CSL): CSL achieved an operating revenue of ₹121 Crores in H1 FY24, representing a substantial 23.2% year-on-year growth.
- Receivable Days: The company reported receivable days at 103 days.
- Inventories: As of September 30, 2023, the company’s inventories, including in-transit inventory, were at ₹337 Crores.
Business Highlights (H1 & Q2 FY24):
- Emerging Markets: Caplin Point’s unique end-to-end business model in Latin America continues to drive consistent growth in both top-line and bottom-line results. The company’s expansion into branded generics and new product launches in various markets have been key drivers of growth.
- Supply Expansion: The company has started to supply major orders for specialty products in Latin America, serviced using contract manufacturing organizations (CMOs). It plans to transition to its high-potency manufacturing site in the near future.
- API Development: Caplin Point has successfully developed over 80 active pharmaceutical ingredients (APIs) at research and development (R&D) scale, ready for scaling up once the API units become operational.
- Third-Party Manufacturers: The company has partnered with existing and new third-party manufacturers with regulatory approvals to penetrate the key Mexico market, particularly in areas like Penicillin and Cephalosporin range of products.
- US & Regulated Markets: Caplin Steriles USA Inc. was established in Hamilton, New Jersey, and is in the process of obtaining licensing for all 50 states. The company plans to launch over 15 own-label products in the US within the first 12 months of incorporation.
- Product Expansion: The company commercialized its high-speed vial filling line, which is expected to boost revenues in the coming quarters. Caplin Point has filed various niche products with the FDA, including Ready-To-Use Bag products and Emulsion injections. It has further products planned for the coming months, including Suspension Injectables, Emulsion Injectables, Emulsion Ophthalmic, and Plastic Vial injections.
| Subscribe To Our Free Newsletter |