This post is collaborative work between @Anant , @Sanjay_Kumar_E and myself. To understand what follows, please read this post first
We looked at classes of antibiotics in the previous post but missed classification for bacteria, so perhaps that’s a good place to get started.
Classification
Gram staining method is used to classify bacteria into gram-positive or gram-negative based on their cell wall structure and composition. Gram-positive bacteria have simpler cell wall structure thats made of a thick wall of peptidoglycan and appear blue under microscope. Eg. Staphylococcus aureus, Streptococcus pyogenes etc. Gram-negative bacteria have more complex cell wall structure made of thin wall of peptidoglycan surrounded by another lipid membrane and stain pink/red under microscope. Eg. E. coli, Pseudomonas aeruginosa. The reason for bringing this up is to put across the point that the complex cell-wall structure makes gram-negative bacteria relatively less susceptible to antibiotics.
Moving onto some common bacteria and treatments, so you can get an idea of how diverse these things are in terms of what they affect and what treatments exist.
| Bacteria | Causes | Treatment |
|---|---|---|
| Staphylococcus Aureus | Skin infections, pneumonia | Vanomycin, Daptomycin, Linezolid |
| Streptococcus Pneumoniae | Pneumonia, Meningitis, Ear infections | Penicillin, Ceftriaxone, Macrolides (Azithromycin) |
| E. coli | Stomach infections, UTIs | Ciprofloxacin, TMP-SMX, Nitrofurantoin |
| Pseudomonas Aeruginosa | Complex infections in Hospital settings | Ciprofloxacin, Ceftazidime or Carbapenems |
| Clostridum difficile | Diarrhea and Colitis | Metronidazole or Vancomycin |
| M. tuberculosis | TB | Isoniazid, Rifampicin, Pyrazinamide |
| H. Pylori | Peptic ulcers, gastritis | Clarithromycin, Amoxicillin, Metronidazole |
Antibiotic Resistance
Before we move onto WCK 5222, lets first understand antibiotic resistance as this is a significant piece. Resistance can happen due to genetic mutations in the bacteria, gene transfer between species, antibiotic overuse causing proliferation of resistant species, not completing courses and also use of antibiotics in the food chain. The last 3 of these are particularly more prevalent in developing countries.
According to last available statistics from 2019, AMR (Anti-Microbial Resistance) caused 1.27 million deaths around the world. In the US, the 5 most harmful pathogens with serious resistances are
| Bacteria | Deaths |
|---|---|
| S. Aureus | 49100 |
| E. Coli | 34000 |
| P. Aeruginosa | 15300 |
| E. Faecium | 13700 |
| A. baumannii | 13,300 |
The ones highlighted in bold, are where WCK 5222 has proven to be very effective (Incidentally Wockhardt’s Emrok targets Methicilin-resistant S. Aureus and is a recent launch). These specifically happen in hospital settings (say ICU) alongside a organ-transplant, cancers or any other surgery, specially in immunity compromised patients
For the ones of interest to us, these are specific resistances that are known and documented
Beta-Lactams
This class of antibiotics are characterised by the presence of common-structural element which is the beta-lactam ring that looks like this.
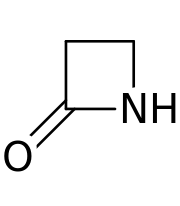
Penicillin is a classic eg. of beta-alactam and you can see this ring in it.
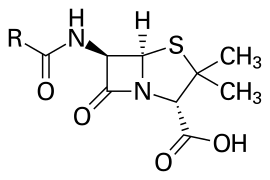
Beta-lactams in common usage are Penicillin (amoxicillin, ampicillin), cephalosporins (cephalexin,cefepime), carbapenems (meropenem) and monobactams (Aztreonam).
These work by binding to PBPs (Penicillion-binding proteins) which in turn weakens the peptidoglycans in the cell-wall due to disruptions in the cross-linking. These being in use for so much for so long has caused resistance mechanisms to develop in bacteria which now produce beta-lactamase, an enzyme that breaks the beta-lactam ring in these antibiotics. By no means is this the only resistance mechanism, although its the most common.
In the previous AMR table containing the 5 pathogens, S. Aureus and E. Faecium are gram-positive while the other 3 are gram-negative. The other 3 are commonly treated with beta-lactams – but due to resistance developing against beta-lactams like carbapenems (imipenem, meropenem) and cefepime, among other things is why these deaths happen today.
BLI (Beta-Lactamase Inhibitors)
Antibiotics have move forward with the introduction of beta-lactamase inhibitors in Sulbactam and Tazobactam in the 80s. However bacteria have evolved as well to produce ESBLs (extended-spectrum beta-lactamases) and Carbapenemases on which Sulbactam and Tazobactams don’t work. So along came Avibactam which was introduced in 2015 and has been used in combination with Ceftazidime (CAZ-AVI marketed as Avycaz in the US and Zavicefta in Europe). Remember this as we will have to re-visit this later when we work out the numbers. A thing to note with these BLIs is that they are not active against all classes of beta-lactamases (Yes, there are multiple classes of beta-lactamases as well with evolved resistances)
BLE (Beta-Lactamase Enhancer)
Wockhardt molecule Zidebactam (WCK 5107) is a novel class of beta-lactamase enhancer (BLE). Zidebactam, in combination with Cefepime (Cefepime-Zidebactam or FEP-ZID)seems to be able to function not just as a beta-lactamase that works against all four classes of beta-lactamases (Class A, B, C and D) but it also modifies the PK/PD of Cefepime. This is the most crucial factor to understand – none of the previously known beta-lactamases do this and this is somewhat of a big deal. Cefepime binds to PBP3 and Zidebactam to PBP2 (while also functioning as beta-lactamse inhibitor) and together, they work more effectively than alone – thereby bringing down the T% > MIC (Remember this from previous post?).
By reducing the amount of time the concentration has to be above MIC, it effectively can also increase the breakpoint MIC for WCK 5222. It can also reduce the dosage requirement of Cefepime, thereby reducing for how long treatment itself is required (which will make this a first-in-class and best-in class drug)
Papers
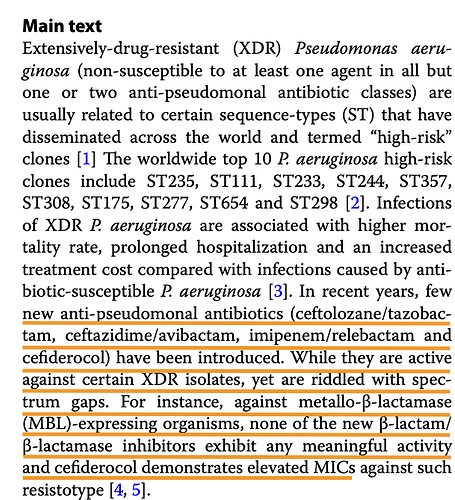
This section can be skipped by most people not deeply interested in the science of it. There are numerous papers published by both Wockhardt folks and independent researchers since 2015 or so that document the effectiveness of WCK 5222 against Pseudomonas Aeruginosa, Enterobacteriales (a class that includes E. coli, Klebseilla) and Acinetobacter Baumannii. Lets go over each of these pathogens
Pseudomonas Aeruginosa
This commonly causes UTI, respiratory tract infections (pneumonia), bloodstream infections (sepsis) and also burn infections. While its treated with beta-lactams like Cefepime or fluoroquinolones like ciprofloxacin (Remember these from last post), Multi-drug Resistance pseudomonas (MDR P. Aeruginosa) is particularly hard to treat. Currently Polymixins like Collistin are used but they are severely toxic.
There are several papers published on WCK 5222 and its effectiveness in treating MDR Pseudomonas Aeruginosa
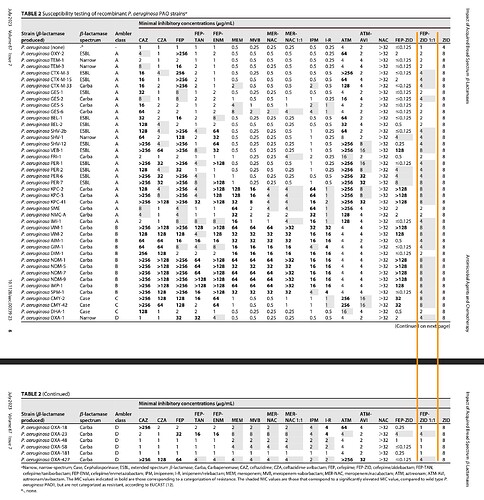
The following table is picked from one of the papers above. Here we can see that FEP-ZID at 1:1 ratio is the only drug that is consistently active against all resistances, be it ESBL or Carbapanem and at low MICs. Others like Meropenem-Nacubactam (MER-NAC) or Cefepime-Taniborbactam (FEP-TAN) are at their breakpoint dilutions against lot of the Carbapenem resistant strains, whereal FEP:ZID 1:1 is several dilutions away from breakpoint due to Zidebactam’s BLE characteristics.
Enterobacteriales
These include E. Coli, Klebsiella etc. and resistance can vary from ESBLs, Carbapanemases to MBL-producing. Pathogenic strains of E. coli that devleop these resistances can be very hard to deal with.
Here are some papers published on WCK 5222’s performance against E. coli
| Who | Where | When | Link |
|---|---|---|---|
| Instituto de Investigación Biomédica A Coruña, Spain | American Society of Microbiology | 15 February 2022 | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8846464/pdf/aac.01676-21.pdf |
| JMI Laboratories, USA | American Society of Microbiology | 27 February 2017 | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5404591/pdf/e00072-17.pdf |
| Center for Pharmacometrics and Pharmacology, University of Florida, USA | American Society of Microbiology | 19 February 2019 | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6496095/pdf/AAC.00128-19.pdf |
| Dept. of Pathology and Lab Medicine, University of Louisville, KY, USA | Antibiotics, MDPI, Basel, Switzerland | 23 March 2019 | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6466586/pdf/antibiotics-08-00032.pdf |
| JMI Labs, USA | Journal of Antimicrobiasl Chemotherapy | 13 June 2022 | https://journals.asm.org/doi/full/10.1128/aac.01432-20 |
| CHINET, China | American Society of Microbiology | 16 December 2020 | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7927829/pdf/AAC.01726-20.pdf |
| CHINET, China | American Society of Microbiology | 12 July 2022 | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9431184/pdf/spectrum.01854-22.pdf |
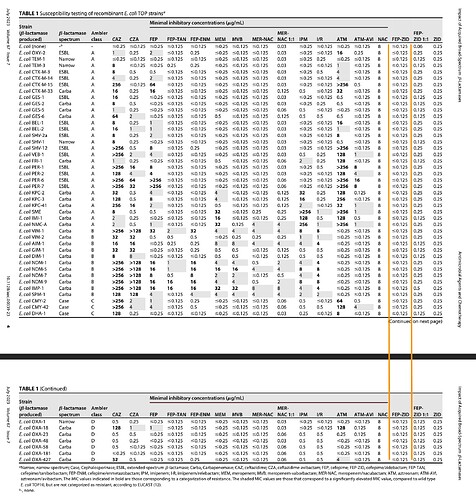
The following table is picked from one of the papers above and it shows that FEP-ZID is active at fairly low MICs against all known resistance strains be it ESBL or Carbapenamase. WCK 5222 shines here too.
While couple of other drugs like MER-NACU or FEP-TAN are also effective, they are not effective against all at consistently lower dilutions. In a hospital setting where time-constraints will limit screening for the pathogens, the broadest, most-effective at lower dilution (away from the resistance breakpoint as we saw in previous post) will tend to be preferred as first-in-line defence.
Acinetobacter Baumannii
This is one of the hardest to treat and spreads in hospital settings. It is resistant to Carbapenems
| Who | Where | When | Link |
|---|---|---|---|
| Wockhardt Research Center | American Society of Microbiology | 27 March 2019 | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6437547/pdf/AAC.02146-18.pdf |
| Center for Anti-Infective Research, Hartford Hospital, CT, USA | Antimicrobial agents and Chemotherapy | 21 December 2018 | https://journals.asm.org/doi/10.1128/aac.01931-18 |
| Dept. of Pathology and Lab Medicine, University of Louisville, KY, USA | Antibiotics, MDPI, Basel, Switzerland | 23 March 2019 | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6466586/pdf/antibiotics-08-00032.pdf |
| CHINET, China | American Society of Microbiology | 16 December 2020 | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7927829/pdf/AAC.01726-20.pdf |
| CHINET, China | American Society of Microbiology | 12 July 2022 | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9431184/pdf/spectrum.01854-22.pdf |
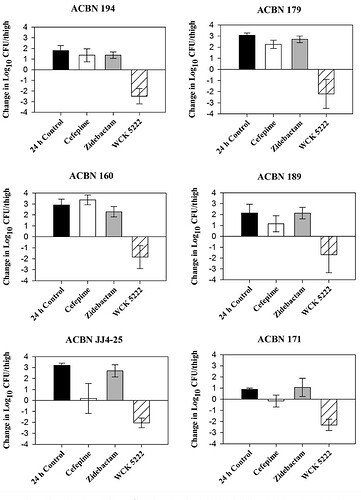
WCK 5222 exhibits a 3-log kill (99.9% if you remember from previous post) in various strains of hard-to-treat Acinetobacter Baumannii (ACBN here). WCK 5222 is consistently shown to be effective in-vivo here against all strains. The 2 to 3-log increase in growth with just FEP shows the resistance A. baumannii has against Cefepime. Remember 1-log kill is sufficient for a FDA approval.
Compassionate Use
There are a couple of papers published on cases of compassionate use that we could find. While the papers above are academic and very promising. There’s nothing more heartening than seeing real-patients on the verge of death being saved by the wonder drug.
Case 1 – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10433839/pdf/aac.00500-23.pdf
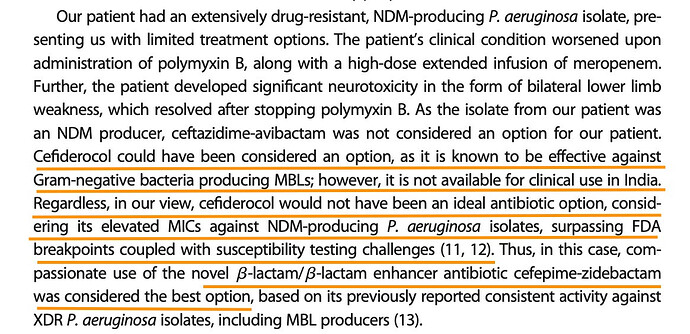
NDM producing Pseuromonas Aeruginosa infection in 18 year old male with T-cell leukemia was cured of it at AIG Hyderabad with WCK 5222.
Some snippets from this paper
Why not Cefiderocol as per the paper. Note that FEP-ZID could go up against Ceftazidime-Avibactam the currently ineffective treatment (CAZ-AVI or Avycaz) and Cefiderocol the new challenger. In India FEP-ZID could be the preferred option against the NDM-producing P. Aeruginosa, going by this paper
The summary from the paper
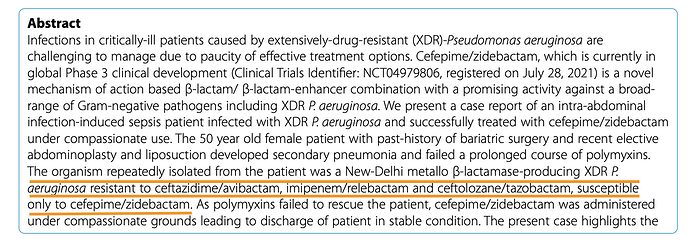
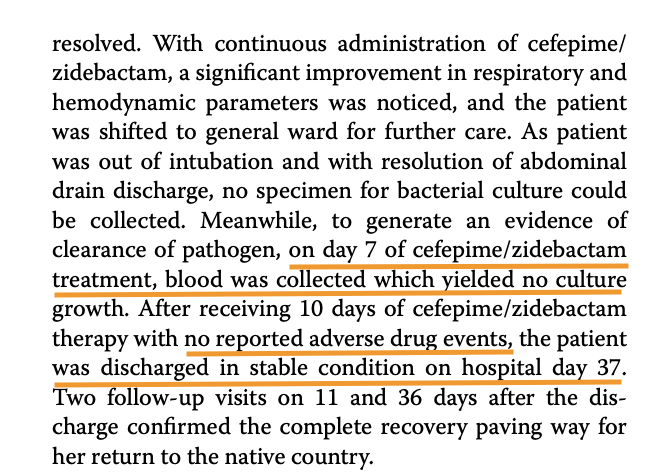
Case 2 – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10324185/pdf/12941_2023_Article_606.pdf
A 50 year old woman from Nepal was treated at Medanta, Lucknow. MBL-producing P. Aeruginosa with A. baumannii were detected in patient.
Multiple drugs were tried on the strain isolated from the patient in this case, prior to treatment
WCK 5222 seems to be the preferred option in MBL-producing P. Aeruginosa
Another happy ending post WCK 5222
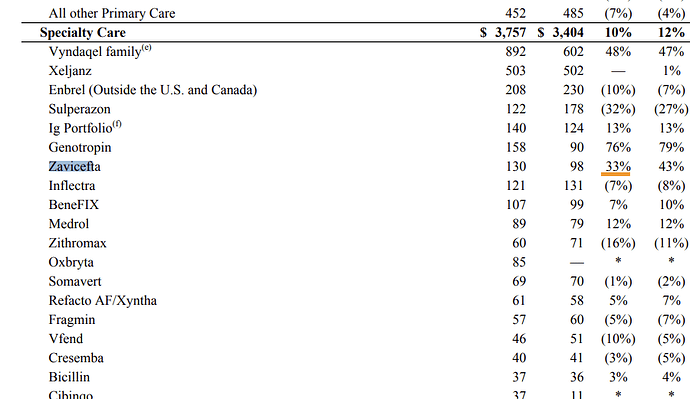
Market Size
From whatever we have seen in the papers, FEP-ZID can go up against CZA (Ceftazidime-Avibactam) sold in US and EU as Avycaz/Zavicefta and Cefiderocol which is a new drug marketed as Fetroja by Shionogi. Both these have shown upwards of 30% growth post Covid which we presume is due to rise in AMR.
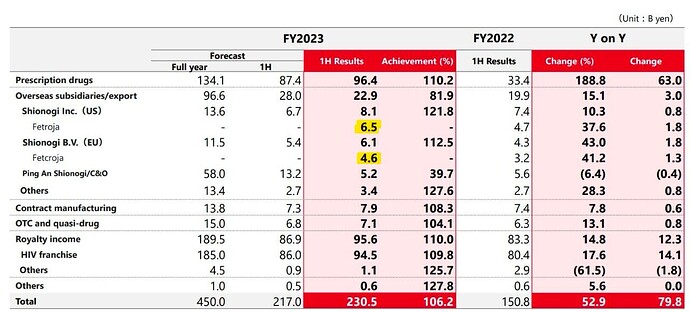
These are sales of Fetroja, as per Shionogi. 11.1 billion Yen in H1 works out to $78m, so annualising, its at ~$160m. Its growing at 38% in US and 41% in Europe.
Side Note: Orchid is going to do Cefiderocol manufacturing for LMIC
Zavicefta has sales of $130m in Q3 ‘23 for Eu+Row as per Pfizer quarterly report.
Assuming similar number in the US for Avycaz and annualising, gives us a market size of $1b for Ceftazidime+Avibactam.
It may not be outlandish to assume $500m market size conservatively for WCK 5222 globally.
If we use a different method to arrive at the numbers – based on avoidable deaths from P. Aeruginosa, Enterobacteriales and A. Baumannii which add up to 64k in the US alone (from the table earlier in this post) and considering a similar number as what Cefiderocol or Avycaz treatments cost – i.e 18k USD/patient gives us a potential $1b in the US – if you discount this to 50% patients at 50% cost – gives us ~$250m in the US very conservatively, With Europe and RoW thrown in, $500m from our earlier assumption doesn’t seem far off. Assuming a 20% royalty on this puts profits for WCK 5222 at $100m or ~800 Cr. If you value this at 15x PAT, this alone can be worth 2x of current market cap.
Other optionalities also exist where FEP-ZID becomes first-line of treatment and replaces some of the existing drugs which can play out over time.
Deals
Some of the deals that have happened in the antibiotics space
| Molecule | Phase | Seller | Buyer | Year | Amount |
|---|---|---|---|---|---|
| Nacubactam | 1 | Meiji and Fedora | Roche (for worldwide rights) | 2015 | $750m |
| Meropenem + Zavicefta + Zinforo + ATM-AVI + CXL | – | AstraZeneca | Pfizer | 2016 | $1.5b + double-digit royalties on sales of Zavicefta & ATM-AVI |
| FEP-TAN | NDA | Venatorx | Melinta | Nov ‘23 | Unknown |
| Xeruborbactam | 1 | Qpex | Shionogi | Jun ‘23 | $140m |
| Enmetazobactam | 3 | Allecra | Shanghai Haini | Dec ‘20 | $78m |
WCK 5222 is comparable to Zavicefta / Meropenem / Nacubactam and is in Phase-3 since Oct ‘22
A Note on Wockhardt Research
This company has spent $350m in research so far and it isn’t by accident that its finding itself with 6 molecules with QIDP (Qualified Infectious Disease Product) status. It has gone about it in the silliest of fashions though with public market capital that is ill-equipped to deal with the risks associated with drug development and discovery. Most innovators work with PE funding or are part of MNCs with deep pockets.
To bring a drug successfully to the market requires the synergistic inter-disciplinary functioning of medicinal chemists, analytics chemists, drug scale-up experts, formulation pharmacists, pharmacologists, microbiologists, toxicologists, vets, pharmacokinetics and metabolism experts, followed by regulatory experts, biostatisticians (to validate efficacy and safety). Wockhardt has spent 12-13% of gross revenue on R&D to sustain this team of researchers for whom the horizon can be a decade or two. Wockhardt has filed 3239 patents and has 810 granted patents. It is a proud moment for us as Indians that an Indian company has gotten this far.
WCK 771 and WCK 2349 have been launched (Emrok and Emrok O) to deal with serious Gram-positive infections, WCK 4873 (Nafithromycin) has successfully completed phase 3 – WCK 5222 is lined up next. There is WCK 4282 in the pipeline that can start phase 3 once WCK 5222 hits the market and WCK 6777 is in phase 1.
Conclusion
Reading too many medical-science papers can drive you mad.
WCK 5222 or FEP-ZID or Cefepime-Zidebactam seems to have very high probability of getting through phase-3 successfully going by papers, as well as the compassionate use cases. At least from a technical standpoint, this seems very promising. The company is probably on the cusp of reaping the rewards for two decades of research. It has to make sure not to goof up marketing and distribution and make sure the licensing deals it gets into capture the value of the product well.
Risks
- Although the phase-3 success looks likely, it is not yet a done deal and so event risk still exists (250 patients are enrolled for phase-3 and WCK 5222 requires 528 to take it to completion – 90% of patients have recovered fully as well so far)
- The company probably isn’t as strong in marketing the drug, manufacturing it (should they decide to go that route) – these can nullify research efforts considering compliance requirements etc. (and the company’s not so great track record)
- Licensing and royalties require good negotiation skills to capture the NPV if going for one-time worldwide rights or good recurring and growing royalties (growing annuities are ideal for us as shareholders)
- Timelines can slip and they already have – WCK 5222 was to hit the market in ‘22 as per company projections in ‘17
- Company has not treated minority shareholders well and has taken larger risks in drug development and discovery – this is something we have to live with
Disc: All 3 of us are Invested
(This post focuses more on WCK 5222 and not on the whole business)
| Subscribe To Our Free Newsletter |