Cefepime+ enmetazobactum(Exblifep) gets a positive opinion for marketing authorization by the committee for medicinal products for human use( CHMP) of the European medical agency. CHMP positive opinion will be referred to the European commission which will grant the final approval in approximately 2 months.
Interestingly, indications mentioned include Hospital acquired pneumonia including ventilator associated pneumonia, even though the original phase 3 study(ALLIUM trial) was done in complicated urinary tract infections. {Exblifep found to have good lung penetration(site of action for pneumonia) in one of the phase2 study}
chmp-summary-positive-opinion-exblifep_en.pdf (163.5 KB)
Current recommendation is based on ALLIUM, a phase 3 multicentre randomized controlled noninferiority double blind trial involving 1034 patients with diagnosis of complicated UTI / Pyelonephritis randomized to receive either Pipercillin-tazobactum ( current standard drug in complicated UTI) VS trial drug Cefepime-enmetazobactum( Exblifep).
EXBLIFEP demonstrated statistically significant superior overall treatment success (clinical cure combined with microbiological eradication) at test-of-care visit compared with piperacillin/tazobactam in cUTI, including AP, caused by Gram-negative pathogens (79.1% vs. 58.9%). Statistically significantly superior results were also observed among patients with infections caused by ESBL-producing pathogens (73.7% vs. 51.5%, respectively) with tolerable safely profile compared to standard drug. Study results were published in original article(behind paywall)
More details from Allecra website about the drug /study trial.
Orchid had licensed the Exblifep to Allecra for development/phase3 trial. Allecra inturn has licensed the product to Advanz pharma for commercialisation within the European union,UK, Switzerland and Norway.
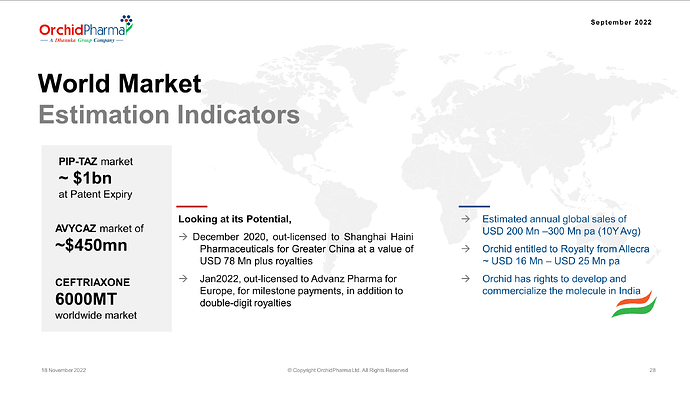
Orchid will get royalty of 6-8% on global sales of Exblifep.
Orchid has indicated that( Nov 22 company presentation) estimated annual global sales of USD 200 mn-300mn per year and expected royalty of 16mn-25mn USD/year. With EU approval it’s likely that US FDA approval also should happen soon and global sales may start from the later half of FY25/26.
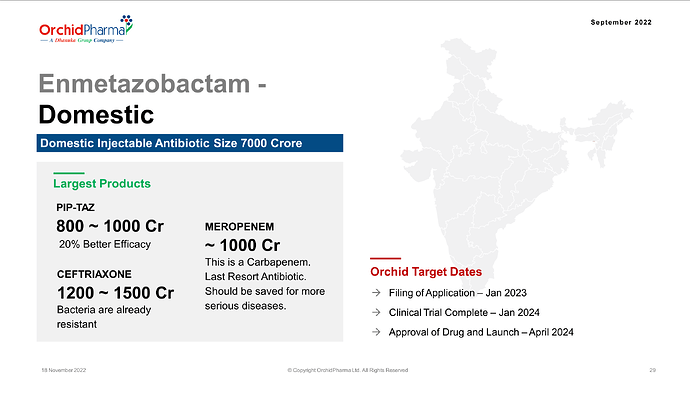
Orchid retained the right to market the molecule in India. current standard drug Pipercillin tazobactum market size in India in around 800Cr.
Disclosure: invested
| Subscribe To Our Free Newsletter |