Wockhardt AR is out and there some details on the other molecules currently under trials in various stages. Some updates on WCK 4282 and WCK 6777 are new
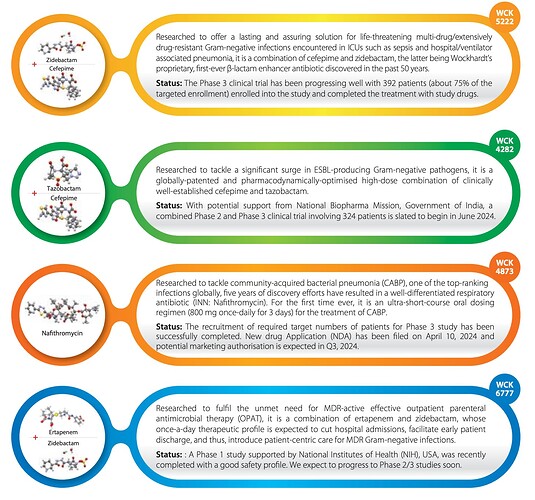
WCK 5222 (Cefepime+Zidebactam) – USFDA Phase 3 with 392 patients signed up and expected to complete in Q4 FY25. Indian Meropenem resistance trial permission from CDSCO says the number of patients is upto 60 and as per company update it is expected to be complete by Nov ’24. Compassionate use so far for 30 patients with 100% recovery rate
WCK 4282 (Cefepime + Tazobactam) – Looks like there’s a combined ph2 and ph3 trial going to get underway in India this month. Permission from CDSCO has additional details. The total subjects could be upto 324. What’s also interesting is that it says potential support from “National Biopharma Mission” – not sure if this indicates financial support for the trial.
National Biopharma Mission in its charter says its funded by GoI and World Bank for development of drugs
WCK 4873 (Nafithromycin) – It looks like India phase 3 is successfully completed and the company has filed NDA with DCGI and it should get approved by Q3 FY25 and be in the market by Q4 FY25
WCK 6777 – (Ertapenem + Zidebactam) – Phase 1 was completed as per US clinical trials. It is highly likely that this will also undergo a combined phase 2+phase 3 trial like WCK 5222.
On the two products which are already launched in India
WCK 771 & WCK 2349 (Emrok and Emrok (0)) – Anti-MRSA drug. 65000 patients have been successfully treated in India and registrations have been filed in 10 countries (Kenya, Uganda, Tanzania, Saudi, Russia mentioned in different parts of AR) and approval is expected in FY25. In India the AR says the use is expanding from CABP to bone and joint infections and febrile neutropenia patients.
Compiling the information into this thread for tracking purpose
Disc: Invested
| Subscribe To Our Free Newsletter |