Generic launch date is based on two considerations (unless an ‘at-risk’ launch):
-
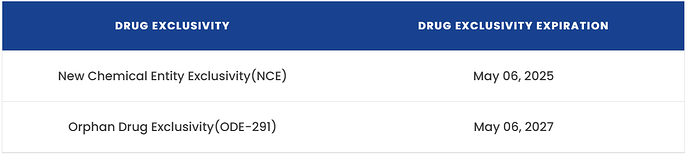
NCE Drug Exclusivity expiration: innovator Novartis has two exclusivities for Tabrecta:
-
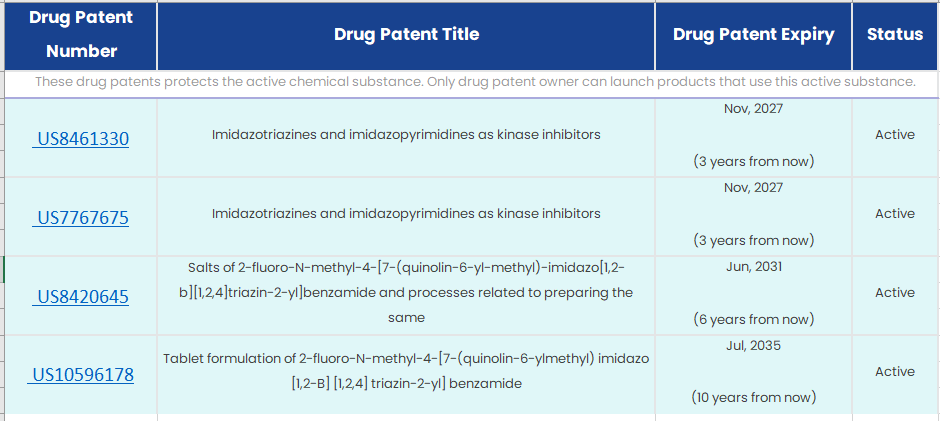
Patent expiration: Based on available data, below is patent expiration summary:
So, best case scenario is Nov’27 for generic launch. Worst case being July 2035
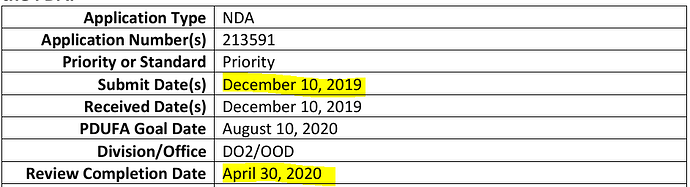
Side note, Novartis had got the approval under the Fastrack route (being Orphan Drug). Again, being Orpah drug, Natco can expect low completion from generics:
Tarun
Disc: No investment
| Subscribe To Our Free Newsletter |