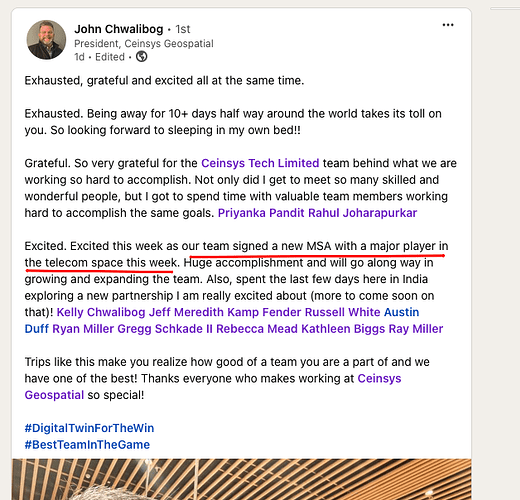
Sun Pharma –
Q2 FY 25 results and concall highlights –
Revenues – 13265 cr, up 10.5 pc
EBITDA – 3939 cr, up 24 pc
PAT – 3040 cr, up 28 pc
Cash on books @ aprox 21000 cr at the consolidated level
Region wise sales –
India sales @ 4265 cr, up 11 pc. Continues to be ranked no 1 in the IPM. Launched 14 new products in India in Q2. Company has 28 brands in top 300 brands in India – highest for any company in India. Also, the company is ranked no1 in 13 different therapeutic segments in India
US sales @ 4290 cr, up 20 pc led by strong growth in speciality products. Launched 2 generic products in US in Q2
EM sales @ 2431 cr, up 3.2 pc
RoW sales @ 1650 cr, down 3.5 pc. Price cuts in Japanese mkts contributed to revenue declines in RoW mkts. Expect this pricing pressure to continue in the next 2 qtrs performance as well
API sales @ 534 cr, up 8 pc
R&D investments @ 793 cr ( at 6 pc of sales ). Out of this 38 pc of money was spent towards speciality products. Company’s R&D pipeline includes 7 speciality molecules in various stages of clinical trials. Guiding for R&D spends @ 7-8 pc of sales for full FY 25
Speciality product sales @ 2373 cr, up 19 pc ( these are high margin products with significant entry barriers )
R&D pipeline –
Liqselvi – Approved in US for Alopecia Areata, awaiting launch
Nidlegy – Filed for approval in Europe for treatment of non-malenoma Skin Cancer
Illumya – for Psoriatic Artritis – undergoing phase 3 trials
MM-II – for pain management in Osteroarthritis – coupled phase 3 trials
Fibromum – for soft tissue sarcomas – undergoing phase 3 trials ( recently in-licensed from Philogen – A European Pharma company )
SCD – 044 – for atopic dermatitis – completed phase 2 trials
GL0034 ( Utreglutide ) for obesity – completed phase 1 trials
The launch of Liqselvi is pending because of an ongoing litigation. Once a favourable judgement is out, the company is ready to launch the product ( ie within a few weeks )
Winlevi – is seeing good QoQ growth in prescriptions in US
Seeing very good traction for Illumetri in China in a short span of time post its launch
Company is very excited about their under trial molecule – Utreglutide
Open to use the cash on Books to acquire speciality assets in the Derma and Derma-Onco space. These are the two long term focus areas for the company. Don’t intend to intend other therapeutic areas as of now
Disc: holding, biased, not SEBI registered, not a buy/sell recommendation