Latest interview on cnbc by Anjan Chatterjee, CMD Speciality Restaurants.he hinted about acquisitions of restaurant chain in near future
https://youtu.be/fFHPCCI8uxk?si=W63aFnOugaOenyHA
Posts tagged Value Pickr
Speciality Restaurants (19-01-2024)
Speciality Restaurants (19-01-2024)
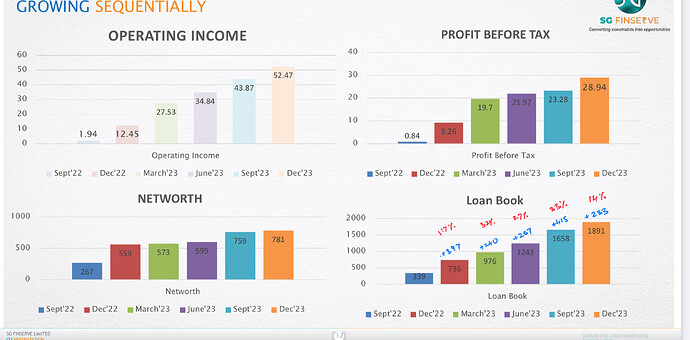
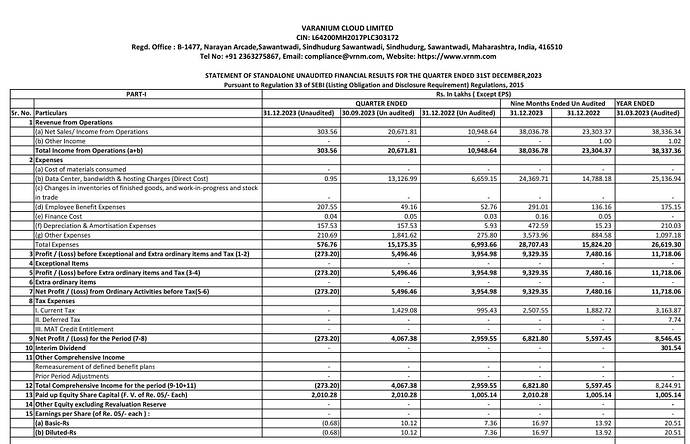
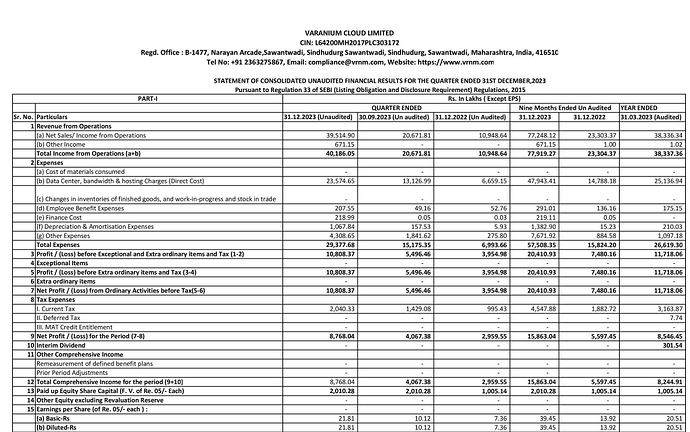
Investors presentation’s with detailed segment revenue https://www.bseindia.com/xml-data/corpfiling/AttachLive/2fba6ff5-3f47-4ad5-a844-be400c108ff9.pdf
EKI Energy is that a story or fact? (19-01-2024)
This business looks suspicious to me as most of the big players exited and retail investors trapped. When big hands out management excepted the mistake and onboarded new auditors. Looks like the party is over now. A question to be asked here is – how can a small company have such a huge number? How can someone do such a basic mistake in accounting? They booked future revenue in current fiscals. A basic business of stove and consultancy projected itself an energy company. It would be great if this community updates this. This company will provide a lot of learning to all.
Disclaimer: I’m invested.
Karan’s small/micro cap portfolio (19-01-2024)
Hi Abhishek,
While the valuation seems a bit on the pricier side, it’s not entirely unreasonable. That could explain why the company hasn’t joined the recent tech rally. Considering current market conditions and my expectation of a lagging US slowdown after interest rate hikes, I’m currently focusing on sectors outside of IT.
Great articles to read on the web (19-01-2024)
Deep sea mining – another gold rush and another ecological disaster in the making?
Gufic BioSciences Ltd (19-01-2024)
Looks like there is significant drop in meropenem prices which is one of the key product for Gufic criticare division. Innovator Pfizer brand Meronem 1gm is available at MRP of 1067.00 which use to be 2-3 times higher few quarters back. Usually innovator brand commands some premium over other generics. When Meronem is available at such price, other generics brand companies also start adjusting their price. Gufic was negotiating with Govt for higher price of 40-50% to launch their dual chamber meropenem. Q3 concall may provide some insights on the same.
Management view from Q2 concall:
Karan’s small/micro cap portfolio (19-01-2024)
The corporate governance issue surrounding CMRL has been a topic of discussion for some time, and similar challenges are not uncommon in the microcap space. While this can raise concerns for some investors, others may prioritize potential growth and strong market positioning.
Previously, I made a similar observation with Associated Alcohol, where governance concerns ultimately impacted the valuation. However, it’s important to remember that each company’s situation is unique.
Cochin Minerals boasts a promising product line and niche market, potentially leading to attractive long-term prospects. However, the ongoing governance concerns remain a factor to consider. Optimizing operational efficiency, as you mentioned with the hypothetical OPM increase, could significantly improve profitability. Ultimately, you must carefully weigh the potential of the business against the identified risks before making your own decisions.
Wockhardt – A story with twist and turn (19-01-2024)
After the phase 3 trial, the company seeks regulatory approval. They can start selling once the regulatory approval is granted.
In the USA, the FDA claims that it takes between 6 to 10 months to approve a New Drug Application (NDA) [1], but frequently it takes more than 12 months. It also takes a long time (anywhere from 2 to 12 months) for the company to prepare the NDA, which is a massive undertaking. So, in total the NDA + review process takes about 8-24 months. The timeline can vary in other countries.
Unlike other drugs, Nafithromycin has been approved as QIDP, so it will be processed under “Priority Review” [2] – we can be sure the review process will take less than 6 months [3]. The total time to market will depend on how fast the company can prepare the NDA. If they take 2 months, then the drug will come to the market within 8 months.
[1] Step 4: FDA Drug Review | FDA
[2] Priority Review | FDA
[3] 21 U.S. Code § 360n