Ah, that image is a post by one of the twitter handle. The content appears to be behind the paywall type. Thus would refrain naming the handle (have high regard for his/her tweets). Cheers.
Posts in category Value Pickr
Virat Crane Industries Limited (VCIL) – sure shot multibagger (22-11-2015)
A big fake ghee syndicate has been arrested last week in Vijayawada, Andhra. The below video explains this, however the video is in Telugu. However, through visuals, one can understand that they sell predominantly Durga ghee to the regions in Vizag, Orissa etc.
The video indicates that genuine brand owners heave a sigh of relief with this large scale fake mafia has been found and stopped.
Stylam- Decent Fundamentals with Cheap Valuation (22-11-2015)
Though debt increased, D/E improved to 1.75 from 1.8
Cupid Ltd – Helping the world play safe! (22-11-2015)
Dear manish962
i have not read this line in the post
"from here on we may expect it to double by end Sept.2016"
and if this is repeated in other threads posts also then its not a healthy practice and must be stopped .
VP CHINTAN BAITHAK GOA 2015: Ananth Shenoy: GENERICS PHARMA PIPELINE BASICS (22-11-2015)
Found an excellent summary of the FDA inspection process.
Recommend to visit the source URL for complete read.
http://www.metricstream.com/insights/FDA-inspections.htm
I have collected the important snippets for reference -
WHO DOES THE FDA INSPECT AND HOW OFTEN?
The Food, Drug, and Cosmetic (FD&C) Act gives FDA the authority to conduct inspections at drug and medical device manufacturing facilities, among other regulated types of facilities. The FDA selects companies to be inspected primarily based on risk . Those companies manufacturing drugs and higher risk devices, top the list. Thus, manufacturers of class III devices, sterile drugs, prescription drugs, newly registered facilities, implantable, life-supporting, and life-sustaining devices, are the primary subjects for inspection.
Facilities having historical significant violations, are also FDA inspected.
For pharmaceuticals and the risk based inspection system, FDA focuses on three types of facilities: sterile drug product manufacturers, those that produce other prescription drugs; newly registered facilities that had not been inspected previously ,and focuses on 3 factor categories: Quality and Product Safety; Facility; Process.
As per the Federal Food, Drug, and Cosmetics (FD&C) Act, domestic drug establishments, and Class II and Class III device manufacturers are to be inspected every two years (surveillance). Foreign manufacturers were inspected on an average of every 9 years. However, due to FDA's budget increase, an increase in manufacturing plant inspections (domestic and international) has resulted. This budget increase is equipping the FDA to add staff and open international offices in major countries . Consequently, foreign inspections, do not have an every 9 year average anymore.
Inspections are also conducted prior to a company acquiring Premarket Approval Application (PMA) and New Drug Application (NDA) approvals, as well as Biologics License Application (BLA) licensing.
FDA QUALITY SYSTEM INSPECTION TECHNIQUE
FDA uses the Quality System Inspection Technique (QSIT) for its inspections. QSIT is based on a top-down approach to inspecting a manufacturer's QS. The technique provides different inspectional levels based on reason for inspection.
In the medical devices industry, QSIT is used by the FDA to assess the firm's QS for compliance with the appropriate regulations and for inspection of domestic and foreign manufactures of medical devices intended for commercial distribution in the United States.
The inspection will assess the firm's systems, methods, and procedures to ensure that the firm's quality management system is effectively established and maintained. The QS inspection should include the assessment of post-market information on distributed devices.
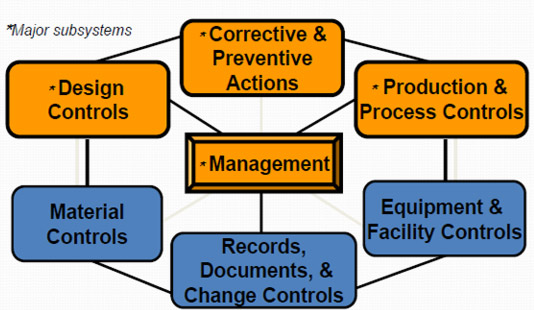
QSIT Devices Overview
The manner in which QSIT relates to the Pharmaceutical Industry, is through the guidance document: "Quality Systems Approach to Pharmaceutical Current Good Manufacturing Practice Regulations". This guidance document defines requirements for all aspects of a firm's quality structure. Firms that comply with the expectations of both 21 CFR Part 210/211 and QSIT, are aligned to the expectations of the referred guidance.
The Center for Drug Evaluation and Research (CDER) defines and groups QSIT into six major systems which consists of:
THE INSPECTION PROCESS IN A NUTSHELL
Here is what happens on the day of an FDA inspection:
• Receptionist activates the designated alert system.
• Company Inspection Team members go into their roles and designated areas.
• FDA provides the company with Form 482 and identification.
• At opening meeting, FDA explains why they are at your site.
• After a facility tour, the FDA inspection team, requests documents and begin to conduct interviews.
• FDA conducts the Exit Inspection Meeting.
• Findings are reviewed and description provided of any violations/deficiencies from current regulations, deviations from company's own procedures and further clarification of misunderstandings can be addressed.
• FDA presents Notice of Observations (Form 483) to management and executive management present at exit meeting with annotations (the latter, if both parties agreed to annotations). The 483 lists inspectional observations. However, it does not represent a final Agency determination regarding your compliance.
• Upon return to local District Office, FDA investigator:
o Writes an Establishment Inspection Report (EIR) (normally, they begin writing it right at the firm's site)
o Forwards report to headquarters and the Agency classifies the inspection
o After headquarter evaluates, you may receive a Warning Letter
WARNING LETTERS
If the inspection has an Official Action Indicated(OAI) classification, the FDA will send a warning letter to the company, describing the manufacturer's violations of FDA regulations and requesting a reply, usually, within 15 working days after receipt of the letter. In such a case, the company will need to reply using the same format as the 483 response, and including documented evidence of corrective actions.
Usually , FDA conducts a follow-up inspection to verify that the appropriate corrections have been implemented and *are effective.
After the FDA has completed an evaluation of corrective actions via follow-up inspection, it may issue a clo*se-out letter.A FEW BEST PRACTICES FOR SMOOTH FDA INSPECTIONS
Companies can ensure a smooth inspection process by following a few best practices:
• Have adequately trained resources with relevant expertise and accountability.
• Get upper management's support on compliance and quality programs.
• Don't underestimate the value of an independent regulatory compliance team and an expert quality assurance team.
• Make sure you have implemented the commitments made from previous inspection.
• Understand your potential quality data sources.
• Implement and assess an effective QS.
• Invest time, designate accountable resources.
• Ensure there is a designated company Inspection Team.
• Ensure proper documentation and records.
• Maintain effective Management Review and CAPA systems.
• Identify true root causes of issues using appropriate problem solving tools.
• In-bed in organizational culture, proactiveness.
• Understand when a product, or quality issue is significant.
• Have defined metric systems to monitor your QS in order to identify trends, gaps, and opportunities.
LEVERAGING TECHNOLOGY TO PREPARE FOR FDA INSPECTIONS
Both for 483s and warning letters, you will need to:
• Put together a project management team and assign accountability.
• Use change and document control systems, CAPAs and other quality systems to implement and monitor the changes needed and to sustain them.
Lycos internet – way to digitalization (22-11-2015)
profit and all is fine if people have confidence on numbers. market perception could be negative also because of relatively complex business. have heard rumours about management being opaque with numbers etc. whats your view on that
Associated alcholols & breweries ltd (22-11-2015)
After seeing the half yearly result there is following points
For growth
3 year sales / profit growth > 7 yr sales / profit growth this will show that a longer period time the growth is sustainable and there is no negative growth in 7 years
For returns
Average ROE 3yr > 12 and TTM ROE > 17%
AVERAGE ROA 3yr = 10 and TTM ROA > 13.5%
For capital
Average ROCE 3 yr > 11.5% and TTM ROCE > 16%
ROIC > 18%
Days receivable in outstanding < 40
Inventory days < 40
Current ratio > 1
Debt management
Debt< Debt 3 yrs back DER < 0.7 Z SCORE > 2.9
Capex growth
Net block > Net block 3yrs
Promoter
Holding increased in last 3 yrs by open market > 5.5
No dilution no bonus no pending warrant no preference capital
Sector
Has problems with government but potential to grow
Promoter quality
Not fully known but no history of non compliance no pending investor compliant and dividend started
No web site
Now as regards the remuneration part I feel that the three people put together that is Prasanna Kumar Sushil Kumar and Ashish Gadia are taking a major chunk nearly > 60%
But the good thing is they have declared it in the PL and even after huge employee cost they are able to show adecent profit in the Pl the cash flow has improved the loan and advance is reduced
The bank grantee of 30 cry to third party has to be understood
And finally as regards the investment made by them in Narmada distillery and Mount Everest has to be clarified
Finally as retail investors we should understand that when HNI like Ayush Anil Kumar Goel dolly Khanna make some investment there financial capacity to hold there ability to cut losses there timing and long term view above all there temperament is different from retail investors
As retail investors we shoul thank them for sharing there perspective on a particular company
Now by fy 17 we can finally say that this is a valuable find if same growth is maintained and promoter clarity comes that I think Ayush has nicely summed up as work in progress watch it for growth and any dieviation
Thank you
Varun 2020 portfolio – 2 strategies (22-11-2015)
Finally started flirting with Camlin Fine sciences and Kitex Garments.
Will look to turn either of them into investments in next 2 days as after that I am going for short vacation to Singapore.
Will invite your views on above stocks. Even Elgi equipments started to look interesting.
Varun 2020 portfolio – 2 strategies (22-11-2015)
Hi
Portfolio Update
Due to some finance requirements have to part away with few stocks like Rallis India, NRB bearings and Gati. While the first two have given very good returns with NRB clocking over 200% the same wasn't the story with Gati. Exited Gati with some loss.
In the meanwhile have steadily increased allocation to few stocks in downturns
Axis Bank
Marico
Atul Auto - On Diwali Day
Have reduced some positions in
KPIT at 166 and Care at 1300 to finance above increased allocation
Happy Investing
Diversified Portfolio with growth target of 18-20% CAGR (22-11-2015)
Hi All,
Thanks for few suggestions.
I can understand that, ValuePickr forum mainly looks for small caps and mid caps which can generate 20-25-30% CAGR on annual basis. Reason for having large caps in my portfolio is to protect the downside considering that I do not have very long time frame for my financial goals.
Also, I have learnt in the past that, even such a large cap oriented portfolio can give returns of up to 25% CAGR if stocks were bought when undervalued and sold when get overvalued. I have always followed a stringent process for this and it has worked quite well.
Reason for TCS being high in portfolio is due to investments since IPO days, and comfort with the management, vision and ability to grow in tough conditions. Also, I understand that, now onwards it will not give me 20% CAGR returns hence have reduced it from 25% to about 14%, and may reduce it further. TCS is a high ROE/ROCE business with good cash flows and good dividend yield.
Mutual Funds can generate returns close to this, but I do not have control on the portfolio, and they keep holding overvalued stocks for a long time. I have more control on the portfolio and can follow my own style of investing. I do have separate MF portfolio.
I am slowly learning the art of identifying small caps which can give 25% CAGR returns and hence there are some quality small caps in the portfolio now. Due to tendency of not overpaying for some of the quality names I do not have Page, Gruh, Repco, Ajanta in the portfolio.

