BJP’s electoral strategy includes year-round worker engagement, early election preparations, and well-timed leadership changes. They utilize ground intelligence, appoint in-charges ahead of time, and efficiently manage internal dissent. These tactics have helped BJP maintain power in various states and outperform rivals.
Dr. Reddy’s Lab – Transformation Journey (19-10-2024)
Quantitative analysis of the company:
Profit & Loss Statement:
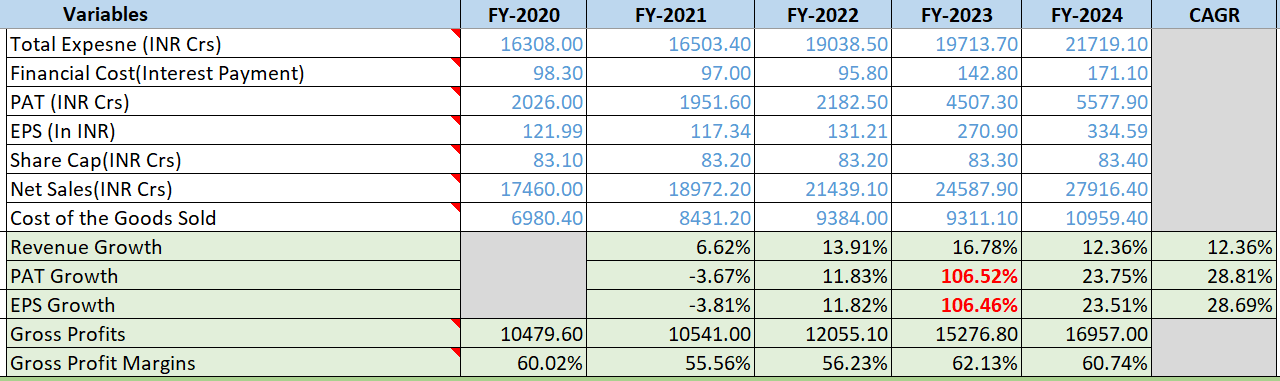
- Revenue CAGR: 12%
- PAT CAGR: 28%, with consistent year-over-year improvement
- EPS CAGR: 28%, also improving year over year
- Gross Profit Margins: Steadily hitting 55+% YoY
There is a spike in PAT & Earning growth in 2023 due to a combination of an increase in Other income, Foreign exchange gain and a reduction in tax
Other Income contributions were Rs.1055.5cr in 2023 vs Rs.484.4cr in 2022.
Details: During the year ended 31 March 2023, the Company entered into a Settlement Agreement with Indivior Inc., Indivior UK Limited and Aquestive Therapeutics, Inc Pursuant to the agreement, the Company will receive payments totaling U.S.$ 72 by 31 March 2024. The said agreement resolves all claims between the parties relating to the Company’s generic buprenorphine and naloxone sublingual film including Indivior’s and Aquestive’s patent infringement allegations and the Company’s antitrust counterclaims.The Company recognised the present value of the amount receivable at `5,638 (U.S.$ 71.39 discounted to present value) on the date of the settlement as other income.The aforesaid transaction pertain to Company’s Global Generics segment.
Balance Sheet:
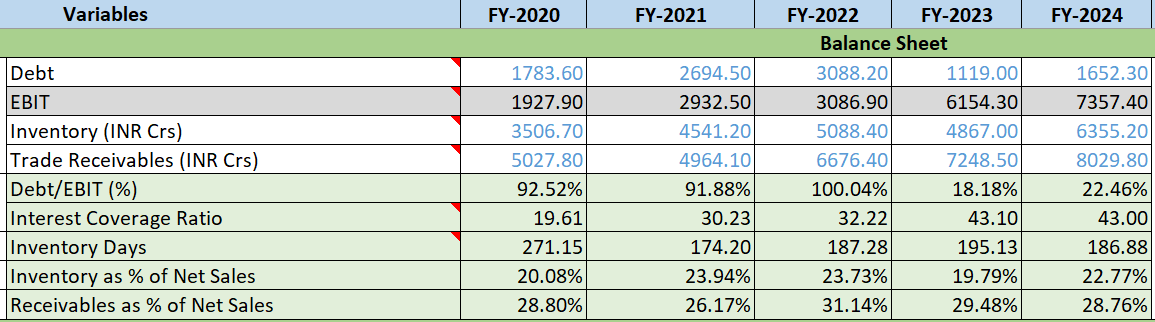
- Debt to EBIT Ratio: Debt/EBIT % is fluctuating but reduced
- Interest Coverage Ratio: Consistently improved YoY, reflecting sound financial management
- Inventory Days: Inventory Days were increasing but only reduced this year and PAT is increasing indicating growth start.
- Receivables as % of Net Sales: Receivables as % of Net Sales is shown downtrend from 3 years, which is encouraging.
Cash Flow Statement:
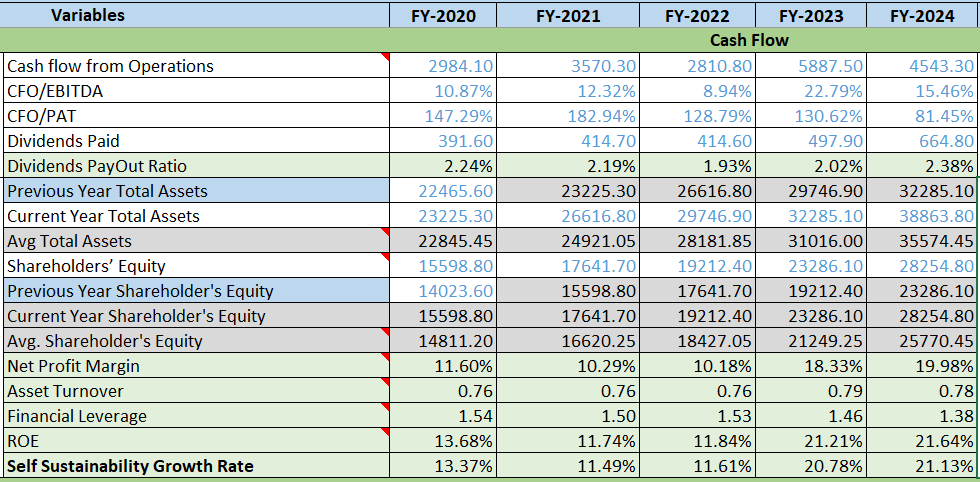
- Net Profit Margin: Increasing Trend from past 3 years
- Asset Turnover Ratio: Consistent around 0.76-0.78 would be good if it starts increasing.
- Financial Leverage: Decreasing, a good sign. would be good if its less than 1.
- ROE: Increasing and hitting above 20% from 2 years
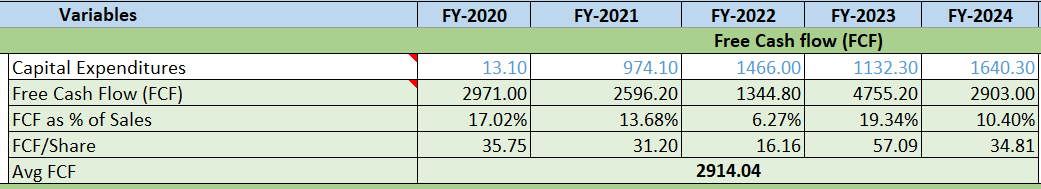
Free Cash Flow:
Positive and increasing FCF and FCF as % of Sales is greater than 5%
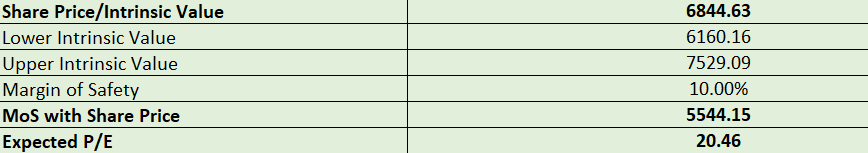
Valuations:
DCF Method considering avg 10% growth for 10 years, 3.5% Terminal Growth Rate, and 8% discount rate.
Conclusion:
Overall, while Dr. Reddy’s Laboratories faces some regulatory challenges, the company remains proactive in expanding its global presence, especially in consumer healthcare and biosimilars. Its diversified portfolio and strategic collaborations indicate a long-term vision for sustained growth. Currently, the stock appears to be fairly valued, if not slightly undervalued. If management delivers on their plans, the revenue from these collaborations, joint ventures, and partnerships is expected to materialize from 2027 onward. In my opinion, DRL represents a solid mid-to-long-term investment strategy with relatively low downside risk, barring any major regulatory issues.
Disclaimer : I am invested, so I may be biased.
Dr. Reddy’s Lab – Transformation Journey (19-10-2024)
Key Highlights on Growth drivers:
Strategic Collaborations, Partnerships and Joint Ventures
-
Nestlé JV: Dr. Reddy’s and Nestlé formed a joint venture (JV) to bring nutraceutical products to the Indian market, leveraging Nestlé’s trusted brands and Dr. Reddy’s well-established commercial capabilities. DRL holds 51% of the JV’s share capital, with Nestlé holding the remaining 49%. The company expects to receive royalty payments from the JV as part of its consumer healthcare strategy.
-
Agreement with Junshi Biosciences: The company signed an agreement with Junshi Biosciences to introduce their novel oncology molecule, Toripalimab, in India and select emerging markets. Recently, the Subject Expert Committee recommended the approval to import and market Toripalimab in India, with a waiver for Phase III clinical trials. The final approvals are expected in the coming months.
-
Collaboration with the Bill and Melinda Gates Foundation: Dr. Reddy’s has partnered with the Bill and Melinda Gates Foundation (BMGF) to manufacture and supply DMPA-SC, a long-acting, self-administered contraceptive injection. This product will empower women by providing more control over their reproductive health, aligning with the company’s goal to make healthcare more accessible and affordable for vulnerable populations. This initiative also supports the UN Sustainable Development Goals (SDGs) for 2030.
-
Sanofi Partnership: An exclusive partnership with Sanofi to market and distribute their vaccine brands in India has elevated DRL to the second-largest player in the vaccine segment.
-
Bayer Collaboration: Dr. Reddy’s partnered with Bayer to distribute Vericiguat, a heart failure management drug, in India. This partnership helps the company expand into Tier-I and Tier-II cities, strengthening its presence in the chronic segment.
-
Pharmazz Collaboration: Dr. Reddy’s signed an agreement with Pharmazz to market Centhaquine (Lyfaquin®) in India, a promising treatment for hypovolemic shock.
-
Amgen Partnership: Strengthening its collaboration with Amgen, Dr. Reddy’s will bring romosozumab (Evenity®), an osteoporosis treatment, to India.
-
MenoLabs Acquisition: Dr. Reddy’s acquired MenoLabs, a women’s dietary supplement brand in the U.S., enhancing its self-care and wellness business.
-
Nerivio Expansion: Following the successful launch of Nerivio®, a drug-free migraine management device, in India, Dr. Reddy’s extended the product to Europe (Germany) and South Africa and is now available in five countries: India, Germany, Spain, the UK, and South Africa.
-
China Market Growth: The company is actively expanding its presence in China, consistently submitting 14-15 products annually, with several interesting approvals.
-
Takeda Partnership: Licensed Takeda’s novel gastrointestinal drug, Vonoprazan, for commercialization in India.
-
Novartis Pharma: Partnered with Novartis to distribute two of their leading anti-diabetes brands, Galvus® and Galvus Met®, in the Russian retail market.
-
Ingenus Pharmaceuticals: Obtained exclusive rights to commercialize Cyclophosphamide Injection in the US.
-
Alvotech Collaboration: DRL is collaborating with Alvotech for the commercialization of their denosumab biosimilar in the US, as well as in Europe and the UK.
Driving Growth Through Innovation and Partnerships
-
U.S. Product Portfolio Integration:
The U.S. generic prescription product portfolio acquired from Mayne Pharma was successfully integrated into Dr. Reddy’s operations this year. -
COYA 302 Partnership:
Dr. Reddy’s entered an exclusive agreement with Coya Therapeutics, a U.S.-based biotech company, for the development and commercialization of COYA 302, an investigational combination biologic for the treatment of Amyotrophic Lateral Sclerosis (ALS). -
Biosimilars and Biologics Pipeline:
Dr. Reddy’s is working on a robust pipeline of biosimilar products, including Abatacept and Rituximab. Although their Rituximab biosimilar candidate received a Complete Response Letter (CRL) from the USFDA due to outstanding issues, the company is actively addressing these and expects approval in the next fiscal year. -
Innovative Treatments in Development:
Dr. Reddy’s has launched new products in the U.S., including Treprostinil and Regadenoson Injections, as well as multiple other drugs acquired from Mayne Pharma. Additionally, the company is advancing oncology and autoimmune disease treatments.
Expanding Consumer Health and OTC Businesses
-
Global OTC Expansion:
Dr. Reddy’s is expanding its presence in the OTC wellness space with the relaunch of Premama® in the U.S. and the acquisition of MenoLabs®. Additionally, they entered the UK consumer health market with Histallay®, an anti-hay fever medicine. -
Nicotine Replacement and Pain Relief:
Dr. Reddy’s continues to build on its global consumer healthcare portfolio, with a focus on nicotine replacement therapy, pain relief, and women’s health products. Dr. Reddy’s is acquiring a leading brand in Nicotine Replacement Therapy (NRT), Nicotinell®, along with three other brands: Nicabate®, Habitrol®, and Thrive®, covering multiple global markets. These acquisitions provide a strong presence in Europe, Canada, Australia, and Japan, while also offering expansion opportunities in emerging markets. Dr. Reddy’s NRT business won a major tender from Brazilian health authorities, marking significant growth in the region. which has a strong presence in over 30 countries. -
Strategic Licensing Agreements:
The company entered into various strategic agreements, including a deal with Tenshi Kaizen to launch Loratadine for the private label OTC business and a collaboration with Mark Cuban Cost Plus Drug Company to provide access to essential medications for Wilson disease patients.
Dr. Reddy’s remains focused on limited competition drugs, with a particular emphasis on injectables and biosimilars, which are expected to drive performance in key markets. The company’s Active Pharmaceutical Ingredients (API) business continues to play a vital role, not only supplying external partners but also supporting its own generic business. This backward integration presents a cost advantage, helping DRL maintain a strong margin profile. The company is further enhancing its backward integration efforts to continue supporting margins.
Dr. Reddy’s is yet to resolve the anti-trust division investigation by the US DoJ concerning price-fixing allegations. Additionally, the company is involved in antitrust lawsuits related to the settlement of patent litigations for Revlimid, which are still being monitored, and the outcomes remain uncertain.
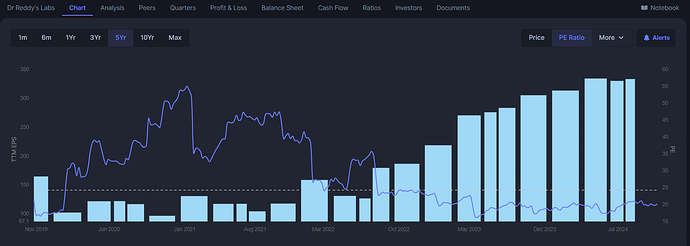
Dr. Reddy’s growth in the US market will continue to be driven by its ability to launch new products and expand its specialty and complex generics portfolio. R&D spending has increased, with 8.2% of sales allocated to research and development in FY24, up from 7.9% the previous year. The increase is primarily due to a higher number of filings and ongoing development efforts in complex products, biosimilars, and small molecules across various markets.
R&D Spending: Approximately 60% of R&D expenditure is allocated to small molecules, with 20% directed to biosimilars and the remaining 20% supporting APIs and other strategic initiatives like in-licensing.
Capex: DRL’s Capex spending focuses 75% on expansion, while the remainder supports maintenance and digital investments.
Dr. Reddy’s remains focused on limited competition drugs, with a particular emphasis on injectables and biosimilars, which are expected to drive performance in key markets. The company’s Active Pharmaceutical Ingredients (API) business continues to play a vital role, not only supplying external partners but also supporting its own generic business. This backward integration presents a cost advantage, helping DRL maintain a strong margin profile. The company is further enhancing its backward integration efforts to continue supporting margins.
DRL’s Aurigene Pharmaceutical Services has inaugurated a state-of-the-art biologics facility in Genome Valley, Hyderabad, dedicated to Contract Development and Manufacturing Organization (CDMO) services. This facility will primarily focus on R&D activities, which aligns with the company’s broader strategy of developing biologics and specialty drugs. Aurigene Oncology Limited achieved promising results in its Phase 1 study for India’s first novel autologous CAR-T cell therapy for multiple myeloma. The Drugs Controller General of India (DCGI) has approved moving to Phase 2 of the trial.
Chirotech Technology Limited, UK: The company dissolved its step-down wholly-owned subsidiary, Chirotech, which had no material impact on the business or financials.
Licensing Agreement with Gilead: DRL signed a voluntary licensing agreement with Gilead Sciences to manufacture and commercialize Lenacapavir in India and other countries.
New Subsidiary in Denmark: Dr. Reddy’s Denmark ApS was incorporated as a wholly-owned subsidiary, marking further international expansion.
Aurigene Oncology Limited (AOL): The company invested ₹2,08,62,912 in Clean Renewable Energy KK 2A Private Limited (CREL), acquiring 26.99% equity in the company.
Rituximab Biosimilar: DRL received a positive opinion from the European Medicines Agency (EMA) for its proposed Rituximab biosimilar. Applications for the biosimilar are also under review by the USFDA, EMA, and MHRA
Bevacizumab (Versavo®): Earlier this year, DRL launched Versavo®, its first biosimilar product in the UK, marking a milestone for the company’s presence in biosimilars.
Product Launches and Pipeline Expansion
In Q1 FY25, DRL made significant progress in expanding its product portfolio:
- NA Generics Business: Launched three new products.
- European Markets: Introduced 12 new products.
- Emerging Markets: Launched 17 new products.
- Indian Market: Brought 13 new products to market.
- Global Filings: Completed 22 global generic filings, including one ANDA in the US.
While the company will rely less on mergers and acquisitions, it will focus more on collaborations, licensing, and acquiring assets or rights to specific assets when needed. DRL is actively building its growth strategy for the years 2026 and beyond, leveraging its strong financial position to make long-term investments in innovation and growth.
This holistic approach is expected to provide DRL with the diversification needed to offset the eventual decline in Revlimid revenues and support the company’s continued growth across geographies and product lines.
Dr. Reddy’s Lab – Transformation Journey (19-10-2024)
Hello Community,
I would like to share some key insights from recent developments at Dr. Reddy’s Laboratories Limited, as it’s been a while since we had a detailed update. Below are highlights from the AR 2024, AGM, Q1 FY25 Results, and the latest credit rating report.
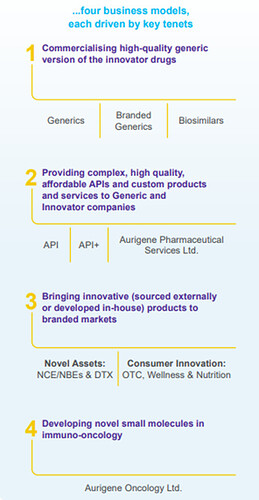
Business Divisions and Products:
DRL provides a wide range of pharmaceutical products and services, including:
- Generics
- Active Pharmaceutical Ingredients (APIs)
- Custom Pharmaceutical Services
- Biosimilars
- Differentiated Formulations
The company is organized into three divisions:
- Global Generics– Contributed to 88% of revenues in FY2024.
- Pharmaceutical Services and Active Ingredients (PSAI)– Contributed 11%.
- Others – Made up 1% of revenues.
Key therapeutic areas include treatments for the central nervous system, gastrointestinal issues, oncology, cardiovascular diseases, and pain management. DRL’s primary markets are the U.S., India, Western Europe, Russia, and CIS5 nations.
Manufacturing and R&D:
API Manufacturing Facilities: DRL operates nine facilities, including six in India, one in Mexico, one in the U.S., and one in the U.K.
Formulation Manufacturing Facilities: The company has 13 plants in India, and one each in the U.S. and China.
Biologics Facility: DRL operates with one biological facility in India.
R&D Centers: It has eight technology development and research centers worldwide, supported by a team of 3,335 R&D scientists.
Product and Regulatory Achievements:
New Product Launches: DRL has launched 181 new products globally, with 20 in North America, 42 in Europe, 106 in emerging markets, and 13 in India.
Drug Master Files (DMFs): It has filed 133 new DMFs globally, including 11 in the U.S. The cumulative global DMFs stand at 1,537, with 251 in the U.S.
Regulatory Filings: DRL has filed 241 dossiers, including Abbreviated New Drug Applications (ANDAs) and New Drug Applications (NDAs). Out of these, 86 filings are pending approval, comprising 81 ANDAs and 5 NDAs. Of these, 50 are Para IV filings, and the company believes 24 hold “First-to-File” status.
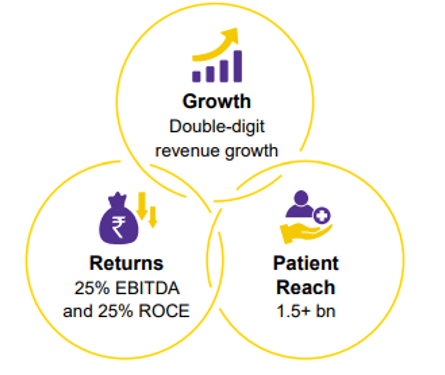
Company Value creations and focus
DRL aspires to generate double-digit revenue growth, 25% of EBITDA & ROCE, and to serve 1.5Bn patients in the next 5-6 years.
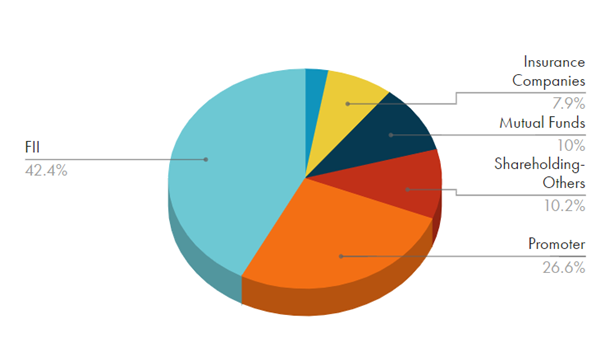
Share Holding Pattern: Public Holding is 10%.
In FY2024, DRL was ranked as the 8th largest generic pharmaceutical company in the U.S. by sales. Globally, it was ranked 11th in India and 15th in Russia as per IQVIA data. The company has grown its market share in several products and improved its ranking in key therapeutic areas across markets.
Aurigene Oncology Limited (AOL)
DRL’s wholly-owned subsidiary, Aurigene Oncology Limited (AOL), is focused on discovering and developing novel, best-in-class therapies for cancer and inflammatory diseases, further expanding DRL’s reach in cutting-edge medical research and innovation.
Over the last four decades, DRL has transformed from a company focused on APIs to one that is a leader in formulations, biosimilars, consumer healthcare, and digital therapeutics, positioning itself for continued success in the evolving global pharmaceutical landscape.
I will be sharing additional key highlights in future posts, focusing on the factors driving growth, the identified risks, and the quantitative aspects of the company.
After LG Nod, J&K CM Omar Abdullah to meet PM Modi for statehood restoration (19-10-2024)
Lieutenant Governor Manoj Sinha has approved a resolution from the Omar Abdullah-led cabinet, urging the central government to restore statehood to Jammu and Kashmir. Abdullah emphasized the importance of reclaiming J&K’s rights and strengthening democracy, including protecting journalists and addressing media accreditation issues.
Frozen Waffle Products Are Recalled Over Listeria Risk (19-10-2024)
Some products were sold under the brands of major retailers like Kroger, Price Chopper and Walmart. No illnesses so far have been linked to the waffles.
Zomato – Should you order? (19-10-2024)
Why do only a few vendors, like Moonstone Ventures LLP and Superwell Comtrade Pvt Ltd, dominate sales on Blinkit, especially considering that they operate 550-600 dark stores across India and that their key management personnel are connected to Zomato’s KMP? What percentage of profit does Blinkit contribute to these companies? How might this create opportunities for revenue leakage?
Disc: I’m not invested, but I’m curious about the supply chain operations and profitability of quick commerce.
Vivek Gautam Portfolio (19-10-2024)
John (Jack) Bogle founded Vanguard, building it into one of the world’s largest and most respected investing institutions on the planet Championing low-cost index fund investing, he’s enabled millions to build long term wealth Here are som pearls of wisdom from the man himself
- ‘Don’t look for the needle in the haystack. Just buy the haystack!’
If you don’t enjoy stock research, or you’re new to investing and want a minimum effort approach, just buy low-cost index funds
-
‘Investing is not nearly as difficult as it looks. Successful investing involves doing a few things right and avoiding serious mistakes.’ From my years of investing and coaching others, the biggest mistake I see people make is overcomplicating their strategy. Keep it simple!
-
‘Time is your friend; impulse is your enemy’
Invest with a long-term outlook of multiple decades and avoid buying the hype or out of FOMO. Hold strong businesses/index funds over the long term and you’ll do better than most.
- ‘Where returns are concerned, time is your friend. But where costs are concerned, time is your enemy’
Compound interest is a beautiful thing. But every day it’s not working for you, it’s working against you. Particularly if you have debts
- ‘If you have trouble imagining a 20% loss in the stock market, you shouldn’t be in stocks’
If you could go back in time and pick the best-performing stocks of the last 5 years, your portfolio would suffer several 40% dips. Volatility is the price you pay for high returns.
‘Learn every day, but especially from the experiences of others. It’s cheaper!’
- ‘Fund investors are confident that they can easily select superior fund managers. They are wrong.’
If you’re going to invest in a fund, make it a passive one. Most active managers fail to outperform the passive funds.
7 Study those who’ve succeeded where you wish to succeed. If someone’s book can teach you in 30 mins what would take 5 years to learn from trial and error, it’s worth the investment!
- ‘The true investor… will do better if he forgets about the stock market and pays attention to his dividend returns and to the operating results of his companies.’
It takes time to master, but always stick to the fundamentals!
- ‘In the long run, investing is not about markets at all. Investing is about enjoying the returns earned by businesses.’
Never forget, investing is buying part ownership in a company. Make sure it’s a company you believe in long term.
-
‘My biggest prediction for the future is that people are going to start looking after individual investors.’ This was an interesting one, because he’s spot on. Investing for retail investors is more accessible and cheaper than ever!
-
‘The stock market is a giant distraction to the business of investing.’ Don’t let the reams of ticker symbols, daily movements, articles and information distract you from why you’re investing in the first place. Stick to your strategy and ignore the noise!
BONUS quote:
‘The historical data support one conclusion with unusual force: To invest with success, you must be a long-term investor.’
Simple facts: most short-term speculators (swing and day traders) lose money. The longer you hold, the more likely you are to succeed!