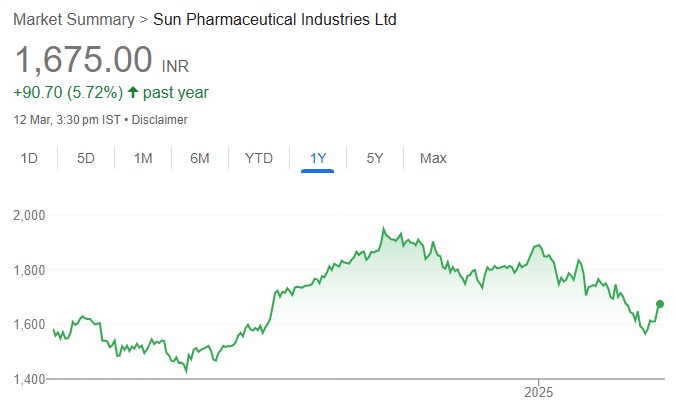
Strengthening Onco Derma portfolio
Sun Pharmaceuticals Industries’ (SUNP IN) acquisition of Checkpoint Therapeutics (CKPT) would give SUNP access to a USFDA-approved novel drug asset, Cosibelimab (approved in December 2024), meant to treat locally advanced or metastatic cutaneous squamous cell carcinoma (laCSCC and mCSCC). Through this acquisition, SUNP intends to deepen its presence in the skin cancer segment; SUNP’s existing portfolio in this space includes Levulan and Odomzo, having filed Nidlegy for melanoma. The acquisition is valued at USD 355mn (USD 400mn including Contingent Value Right (CVR). We estimate peak sales opportunity of USD 250mnUSD 500mn for Cosibelimab. We retain our estimates on SUNP and retain BUY with a PT of Rs. 2,206 based on 35x FY27E EPS.
Cosibelimab can be a USD 250mn-USD 500mn peak sales opportunity
Nearly 2.4mn annual incidences of cutaneous squamous cell carcinomas have been recorded globally, of which, 2%-4% are locally advanced/metastatic cases. Cosibelimab is a USFDA approved drug that is awaiting filing in Europe. The drug would be competing against two other approved treatment options – Keytruda (Merck) and Libatyo (Sanofi) – in the same indication. Competition in the space could intensify with a few other drugs currently undergoing clinical trials. Moreover, Keytruda (pembrolizumab) is also expected to face competition from a biosimilar 2028 onwards. We estimate a 10% market share for Cosibelimab, with a treatment cost of USD 100k per patient, which translates into peak sales opportunity of ~ USD 250mn (assuming 2% of cases as advanced/metastatic cases and 50% treatment rate).
Potential upside to peak sales of Cosibelimab can differentiate on long term data
On the primary endpoint, the objective response rate associated with Cosibelimab stood at 47% and was comparable to Libatyo, but better than Keytruda. There is a possibility for Cosibelimab to differentiate from Libatyo on the long-term benefit. The trend for patients put on Cosibelimab, followed up for long term data, has been encouraging. Based on the most recent long-term data, Cosibelimab demonstrated robust and durable overall response rates (ORRs); in mCSCC and laCSCC, the ORR rates in patients with advanced CSCC– were 50.0% and 54.8%, respectively. Results demonstrated response deepening over time, resulting in higher complete response rates than initially reported during primary analysis. Overall, mCSCC and laCSCC showed complete response rates of 12.8% (7.7% at time of primary analysis) and 25.8% (9.7%), respectively. A clinically meaningful duration of response (DOR) was also observed with Cosibelimab, with the median yet to be reached.
Key details of the acquisition:
arget entity: CKPT is a commercial-stage company focused on novel treatments for solid tumor cancers, notably with its FDA-approved drug UNLOXCYT™ (cosibelimabipdl) for metastatic or locally advanced cutaneous squamous cell carcinoma (CSCC).