Order vacating injunction on Leqselvi a material positive
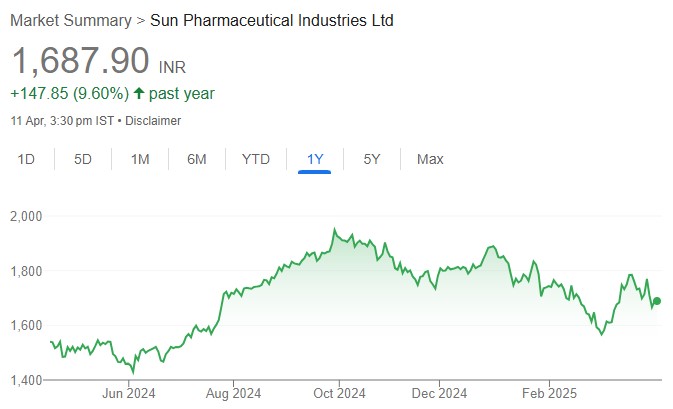
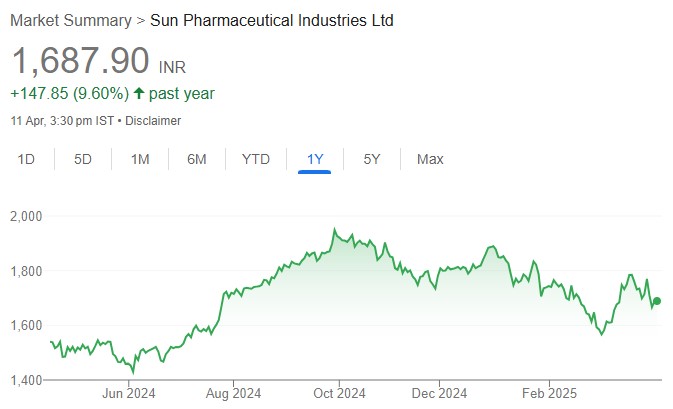
The US Court of Appeals has vacated the preliminary injunction against Sun Pharma’s launch of Leqselvi which was granted by the US District Court of New Jersey in Nov-24. While the decision of the US Court of Appeals now technically clears the way for Sun to launch Leqselvi, we note that the litigation between Incyte and Sun is still under way and a launch prior to the expiry of Incyte’s ‘335 patent (Dec-26) will be an at-risk launch. Sun has stated that it will disclose its launch plans in due course. However, given that our (as well as the street’s) base case is Sun entering a royalty-based settlement with Incyte, the development is a material positive for Sun from a negotiation/potential settlement standpoint. An FY26 launch continues to be our base case, and we have currently factored in US sales of USD40mn/124mn for FY26E/27E from Leqselvi. While Leqselvi’s EPS contribution in FY26E (marginally dilutive)/FY27E (0.5%) is not meaningful, considering the launch-linked costs that we have built in, we expect the Leqselvi opportunity to be as significant as Ilumya is today by FY30 (US + ex-US sales of more than USD500mn, per our estimates). We reiterate BUY.
An FY26 launch continues to be our base case
Incyte’s patent in lawsuit (‘335), the only patent that is currently holding up Sun Pharma’s launch of Leqselvi, expires in Dec-26. With the patent expiry looming and Sun seeking to launch the product at the earliest, the street’s as well as our base case has been that Sun and Incyte will enter a royalty-based settlement in due course. The US Court of Appeals vacating the preliminary injunction is a material positive for Sun from a negotiation/potential settlement standpoint. Incyte already has a royalty agreement with Eli Lilly for Olumiant (Baricitinib), while Sun Pharma has a royalty agreement with Aclaris Therapeutics for Leqselvi. Leqselvi’s relatively late entry vs competing JAK inhibitors (Eli Lilly’s Olumiant and Pfizer’s Litfulo) is not a concern, given its far superior efficacy as well as benign side-effect profiles. We have currently factored in US sales of USD40mn/124mn for FY26E/27E from Leqselvi.
Leqselvi opportunity likely to be as significant as Ilumya is today by FY30
We expect the Leqselvi opportunity to be as significant as Ilumya is today by FY30 (broadly in line with expectations reflected in the contingent voting rights awarded at the time of the Concert acquisition – ~USD500mn in annual sales by CY29). While the addressable market for Leqselvi in US at ~0.3mn patients (0.2% prevalence of Alopecia Areata in US with 45% of cases being classified as severe) is fairly large, we expect Leqselvi to also gain share from competing JAK inhibitors. Sun is also expected to launch Leqselvi in markets outside the US in a phased manner. Beyond FY30, when we expect global sales for the brand to exceed USD600mn, we expect Leqselvi to be a 45%+ EBITDA margin brand. Sun also plans to target 2-3 other indications with Leqselvi.