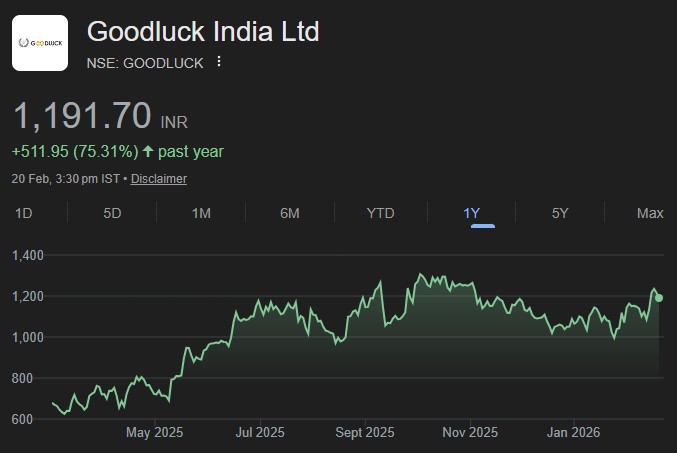
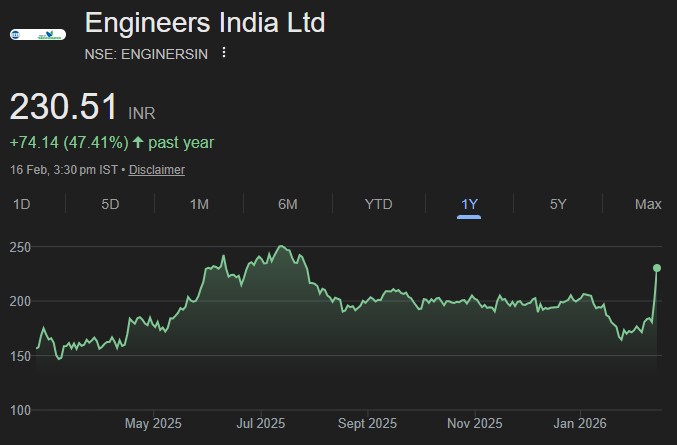
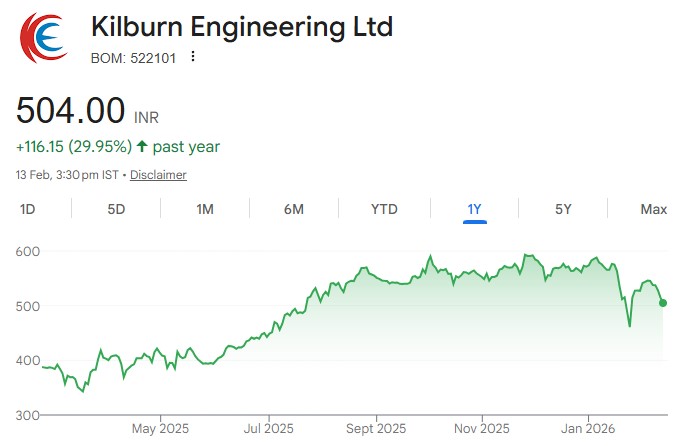
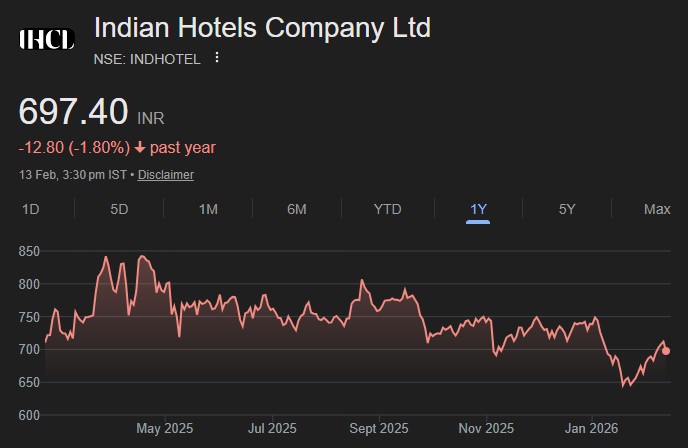
In the Union Budget 2026-27, announcements were made for 7 new bullet train corridors,...
Arjun
SHL has the capability to manufacture oral liquids, tablets, injectables, dry powder & inhaler...
With robust inflows of c.INR 59bn (ex GST) in 9MFY26, ACIL’s backlog strengthened to...
Ahluwalia enjoys a healthy balance sheet and is a net cash company
The 2 acquired subsidiaries – IAC India and GreenFuel Energy continue to drive growth...
EIL is strategically skewing towards consultancy and cost-plus/OBE turnkey contracts, protecting margins from cost...
We expect Revenue and PAT to grow at 29.8% and 32.8% CAGR over FY25-FY28E.
We anticipate healthy double digit net sales growth and operating margin expansion in near...
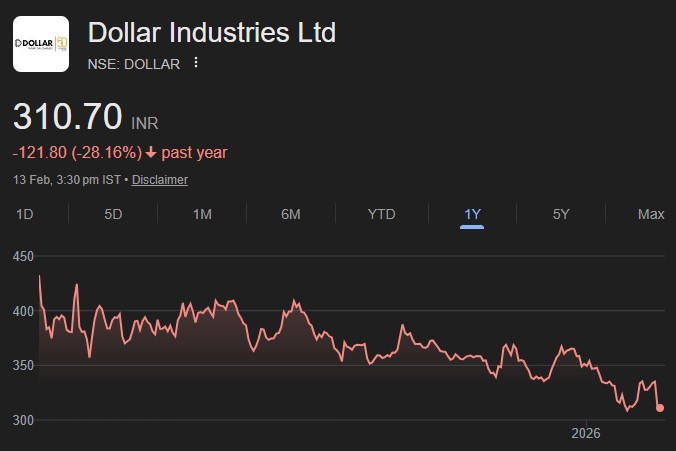
DIL’s strong brand recall coupled with deeper penetration and consumers shifting towards affordable branded...
Management indicated that on-ground demand momentum remained healthy in 3QFY26